Method for Comparison of Leveling in Decorative Acid Copper Plating
Presented here is a test mechanism used to compare and contrast systems for leveling in acid copper plating as well as additives within a system.
by George S. Bokisa, Coventya Inc.
ABSTRACT
Leveling in acid copper plating is a nebulous parameter for quantification. Most techniques require the tester to make subjective determinations based on visual inspections. As the art has matured, it has become necessary to develop techniques that can determine small, incremental improvements in leveling so that comparison of systems can be done objectively. Further, as small changes to leveler molecular structure can greatly impact charge distribution and hence leveling effectiveness, some metric must be put into place to allow differentiation. To this end, presented here is a test mechanism used to compare and contrast systems as well as additives within a system. Testing requires a reproducible substrate, profilmetry, voltammetry and a concise way to present the data.
Keywords: acid copper plating, leveling, quantitative measurement
Introduction
Leveling in acid copper plating is a nebulous parameter for quantification. Most techniques require the tester to make subjective determinations based on visual inspections. As the art has matured, it has become necessary to develop techniques that can determine small, incremental improvements in leveling so that comparison of systems can be done objectively. Further, as small changes to leveler molecular structure can greatly impact charge distribution and hence leveling effectiveness, some metric must be put into place to allow differentiation. To this end, a test vehicle has been developed to compare and contrast systems as well as additives within a system. This testing matrix requires cyclic voltammetry, a reproducible substrate, profilometry and an easy visual presentation of the data.
Traditionally, copper additives are broken into three fairly distinct groups:1
• Carriers (suppressors)
• Brighteners
• Levelers
While many chemical groups can be used, the art has generally evolved into carrier species that are polyglycols.1 Carriers form a film on the cathode and increase the potential necessary for deposition. In simple terms, this film suppresses the plating rate and is the reason carriers are also referred to as suppressors.
Brighteners can also be varied in their chemical make-up. Of most use to the industry have been organo-sulfur compounds. More specifically, substituted propane sulfonic acids1 (SPSA). These species react within the carrier film to enhance deposition by stabilizing the copper reduction and promoting amorphous, semi-microcrystalline structures. When used in combination with carriers, brighteners enhance plating rates. It is this plating rate enhancement that allows for quantification using cyclic voltammetric stripping (CVS).
Levelers are much more varied in chemical make-up. Typically classified as either dye (having visible color) or non-dye (essentially clear in solution), most levelers are characterized by at least one quaternary amine moiety. This quaternary amine imparts a positive charge on the molecule, which is in turn then attracted to the cathode, specifically the higher current density locations. This masks the high current location, slowing plating and allowing surrounding areas to effectively plate faster, so that ultimately the plating reaches a single plane. This is the classical explanation for leveling. Since levelers mask plating areas and inhibit plating, they also have a suppressing effect on the plating rate. This too can be characterized by CVS.
It is this wide variety of materials possible for leveling that makes characterization/quantification for improvement a necessity. An approach to differentiation of leveling effectiveness will be demonstrated by a “methodology walkthrough.” Take the case of one particular class of leveling agents well known to the industry, the phenylphenaziniums (PPZ) also referred to safronic dyes. Probably the most researched of these is Janus Green B (Fig. 1), which has been included in patent applications at least as early as 1952.2
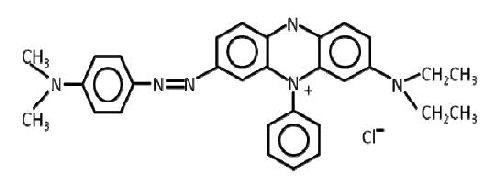
Figure 1 - Janus Green B.
Janus Green B is more correctly known as 3-(diethylamino)-7[{(4-dimethylamino) phenyl}azo]-5-phenylphenazinium chloride. As is evidenced by the structure, the quaternary amine is situated on the PPZ moiety. Given the location of the quaternary amine, it is easy to see that Janus Green B is nothing more than a substituted subset of a lot of possible substituted PPZ. It does not take much of a leap (or a little bit of study of the literature) to understand that the generic structure (Fig. 2) represents a significant number of possible materials that could exhibit leveling tendencies.
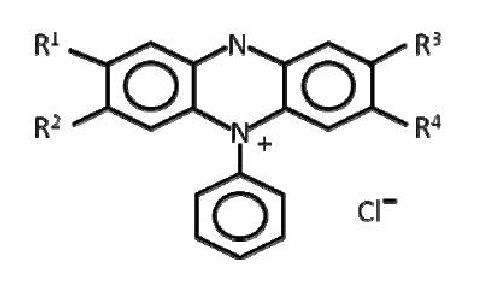
Figure 2 - Substituted PPZ.
Hydrogen, substituted amines, hydroxide, methyl and ethyl groups just start to scratch the surface of possible substitution groups. Further, these substitution groups can profoundly impact the activity of the molecule as a leveling agent.
To begin to sort possibilities, cyclic voltammetric stripping (CVS) is employed to determine relative activity of a leveling candidate. CVS is a common electroanalytical technique used for quantification of organic additives in acid copper plating solutions. A potential is scanned at a constant rate back and forth between negative and positive voltage limits. A small amount of metal is plated onto and stripped off a working platinum rotating disk electrode as the potential is cycled. The additives affect the plating rate. Said rate is quantified by the amount of charge required to strip the metal off the electrode. By holding other variables constant, the relationship between stripping charge, represented as the area under the stripping curve, and the activity of the additive in question can be quantified.
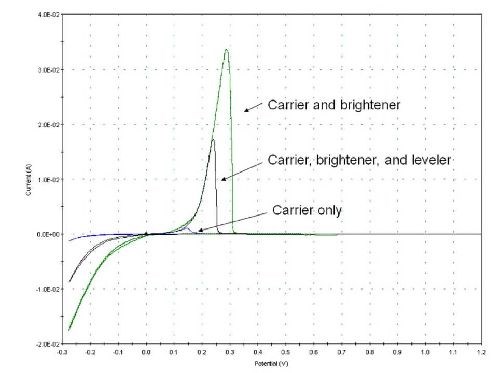
Figure 3 - Additive effects on stripping peak area.3
This is demonstrated on the overlaid voltammograms in Fig. 3. The addition of the carrier component suppresses the plating rate, yielding a very minimal stripping peak. Addition of brightener overtop of the carrier enhances the plating rate, yielding a much increased stripping peak area. Finally, the addition of a leveling agent to the other two once again suppresses the plating rate, reducing the stripping area, but not to the extent of the carrier only.
To compare the efficacy of the leveling candidates, a standard copper plating solution containing 48 g/L copper (as metal), 75 g/L sulfuric acid, and 80 ppm chloride ion was chosen. To this was added 500 ppm of PEG 8000 as a carrier and 60 ppm of a substituted sulfopropyl sulfonic acid (SSS) as a brightener to yield a fairly generic additive background for leveler activity to be measured. All testing was done at a nominal room temperature of 23°C.
The leveler candidates were then made into aqueous solutions containing 0.25 g/L of the active material being considered. The candidate solutions were then added to the base copper solution in 10 ml/L increments and a stripping peak area determined. The response was normalized by taking a ratio of the actual determined peak area divided by the initial peak area, or the “0” leveler starting value. By using the ratio, small day-to-day variations in the instrumental output were minimized.
When the normalized values are graphed vs. concentration, a response curve is generated that characterizes the candidate’s activity. Both the total amount of suppression and the rate at which the total suppression is reached (slope of the line) are indicators of relative leveling strength. Figure 4 is typical of the response for numerous possible leveling candidates.
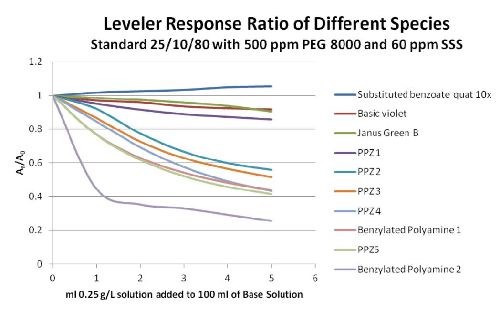
Figure 4 - CVS leveler response.
The testing indicates that, on a weight/weight basis, there are many PPZ type candidates that have more leveling activity than Janus Green B. It also demonstrates that the tested benzylated polyamines tend to have more leveling activity, than the PPZ compounds. Finally, the substituted benzoate quaternary amine, even at ten times the concentration of the other candidates, did not exhibit any leveling activity. In fact, it tended to enhance the plating rate only slightly, making it react more like a weak brightener than a leveler.
Knowing that there are now some viable candidates with leveling activity greater than the standard Janus Green B, functional testing must be done to determine if the activity translates to improved leveling efficacy.
Generally, high current density leveling is not where systems or additives differentiate themselves. Improved leveling tends to manifest best in a system/additive that improves leveling characteristics in low current density applications. This low current density leveling improvement is what will be focused on. Heretofore, low current density Hull cell testing has been the standard method used for evaluation. However, interpreting Hull cell testing to determine incremental improvements is difficult at best and becomes quite subjective. To eliminate the subjectivity, a plating test was designed to truly push the limits of leveling and differentiate both systems and additives.
Each leveler candidate was inserted into a standard additive matrix that included a proprietary additive mixture containing a carrier component, brightener component, and a primary leveling agent that was a quaternary polyamine. The primary leveler is known to give good high to mid current density leveling. The PPZ candidates are secondary leveling agents that will hopefully improve the LCD performance of the systems. The candidates were measured against the leveling effectiveness of other commercial systems available in the market as well as other internal systems. The goal was to produce a system with equivalent performance to the system with maximum leveling with less expensive materials.
Previously, screening was done using a brass panel that was uniformly etched with a persulfate-based solution that produced a very uniform 200-nm roughened surface, which was then used for plating tests followed by profilometry to measure the effective leveling. Testing on the 200-nm roughened panels indicated that a candidate had been found that matched the performance of the benchmark system.
The new candidate was formulated into a package and tested in the field, side by side against the benchmark system on a commercial plating on plastics line. Testing showed the new system to be the equivalent on ABS plastic, but lacking in LCD leveling on polypropylene substrates. The disappointing result necessitated revisiting how the performance testing was done in the lab.
The testing flaw was found by reconsidering the fundamentals of leveling. Figure 5 is a pictorial representation of the leveler theory and the variables with the most impact.
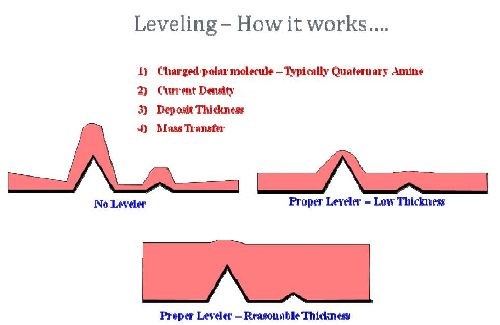
Figure 5 - Leveling fundamentals.
Effective leveling is a combination of charge on the leveler molecule, current density, mass transfer and importantly, deposit thickness. This is important when considering the difference between trying to level a roughened ABS plastic surface and a roughened polypropylene surface. When looking at the relative roughening of the surfaces versus the initial test matrix, it becomes apparent where the test flaw was and what the necessary fix.
The initial testing matrix nearly matched the natural roughness of ABS. It is not surprising then that equivalent performance was garnered on equivalent surfaces. However, the polypropylene roughness is three to four times rougher (Table 1). The test parameters were not sufficiently stringent to differentiate additive performance. To correct that oversight, a new test panel (Fig. 6) was acquired that had an average roughness of 2,200 nm. This provides a much more stringent test and was found to quite sufficiently differentiate the additives.
Table 1 - Substrate roughness comparison.
Substrate | Roughness (nm) |
Initial brass study | 200 |
ABS | 150-250 |
Polypropylene | 600-800 |
New test panel | 2200 |

Figure 6 - New test panel.
This new test panel is a standard molded ABS plastic piece with roughness built into the molding process rather than from pre-plate etching. The part was metalized and acid copper struck with 3 μm of deposit from a non-leveling bath to prepare for test plating. Panel roughness is very reproducible with standard average roughness (Ra) of 2.220 ± 0.005 μm. The panels were plated from the various test solutions at 15, 7.5 and 3.75 A/ft2 for 40 min to simulate a current density gradient that would be found on a complex part. Solutions were air agitated and the temperature was maintained at 25°C.
After plating, all test pieces were subjected to profilometry testing to determine the Ra or the average roughness on the test area. Two of the output profiles are presented in Fig. 7 as a visual example of the roughness deviations.
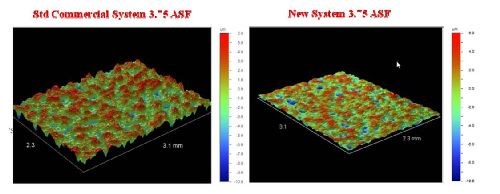
Figure 7 - Profilometry of plated test surfaces.
The roughness for all the current densities and plating times were tabulated. The most effective way found to represent the differentiation of the systems was by comparing the values as a % leveling. This value was calculated by using the following calculation:
((Ra (Start) - Ra (Plate)) / (Ra (Start))) × 100% (1)
The different systems were then grouped by current density and all were presented on a column graph. Figure 8 is the output of the data.
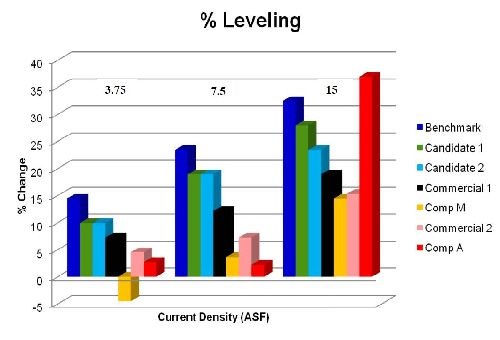
Figure 8 - Percent leveling from tested systems.
The data indicate many conclusions.
1. The benchmark system has very good leveling at all current densities.
2. New leveling candidates 1 and 2 show good promise as compared to benchmark and competitive systems.
3. Comparison M has very poor LCD leveling capabilities. In fact, at 3.75 A/ft2, no leveling takes place and there is actually HCD accentuation.
4. Comparison A has tremendous HCD leveling, but comparatively lacks in the LCD.
5. Present commercial systems outside of the benchmark are very performance competitive with the tested offerings from the competition.
6. Leveling efficacy is current density and time dependent regardless of the chosen system.
With the collected information, a new system has been designed around leveling candidate 1 and field testing is underway to determine long term feasibility. Given the collected data, there is much reason for optimism that field testing will be successful.
Summary
In an industry where many commercial decorative acid copper systems are available and performance differences are minor, a system has been developed to characterize and quantify both new leveler candidates and systems in an objective manner. The use of CVS as an initial screening tool allows for determination of the activity of new possible species. Once materials are identified as active, they can be worked into a system and functionally tested against established technologies and quantifiably compared through use of a standardized panel of sufficient roughness for differentiation of leveling efficacy at varied current densities. The use and charting of the percent leveling allows for an easy to understand, quick visual representation of the deviations between systems, providing an objective determination of leveling efficacy between systems.
1. M. Schlesinger & M. Paunovic, Modern Electroplating, 4th Edition, John Wiley & Sons, Inc., New York, NY, 2000: pp. 67-71.
2. H. Brown & R.A. Fellows, U.S. Patent 2,707,166 (1955).
3. M. Lefebvre, “Electrolytic Copper Bath Analysis for Printed Circuit Board Fabrication,” Dow Electronic Materials Technical Communications, September 2010; www.rohmhaas.com/electronicmaterials/interconnect_technical_site/attachments/AdditiveAnalysis_Electrolytic_Copper_eng.pdf (last accessed 03/16/2013).
Related Content
An Overview of Electroless Nickel Plating
By definition, electroless plating is metal deposition by a controlled chemical reaction.
Read MoreInnovation in Plating on Plastic
Plating on advanced plastics solution offers improved adhesion, temperature resistance and cost savings.
Read MoreTroubleshooting Alkaline Zinc
One of the most common problems that can arise when plating with alkaline zinc is an imbalance of brightener in the solution. In this helpful Ask the Expert article, Chad Murphy of Columbia Chemical discusses how different zinc metal concentrations and brightener concentrations can impact efficiency.
Read MoreLiquid Chrome Vs. Chromic Acid Flake
Contemplating how to continue offering chromic acid services in an increasingly stringent regulatory world? Liquid chrome products may be the solution you’re looking for.
Read MoreRead Next
Education Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read More
















.jpg;maxWidth=300;quality=90)











