Nanostructure of the Anodic and Nanomaterials Sol-Gel Based Materials Application: Advances in Surface Engineering
Porous alumina can be fabricated electrochemically through anodic oxidation of aluminum. This paper reviews sol-gel chemistry and applications, which also offers unusual nanoporous microstructures. The ability to control pore chemistry at different scales and geometries, provides excellent bioactivity, enabling the entrapment of biologically active molecules and their controllable release for therapeutic and medical applications.
by
Xavier Albort Ventura*
Laboratory Electrochemical R&D, Barcelona, Spain
University Politecnic of Catalonia, Barcelona, Spain
Tecnocrom Industrial Cabrera de Mar, Barcelona, Spain
Editor’s Note: A printable pdf of this paper can be accessed and printed HERE.
ABSTRACT
Numerous metals are processed by anodic oxidation. As a result, one can obtain amorphous barrier-type oxides, crystalline barrier-type oxides or amorphous nanoporous oxides. Currently, highly-ordered nanoporous anodic aluminum oxides (AAO) are obtained with various electrolytes to form nanostructures with a range of geometrical features. This material can serve as a template for nanofabrication of variety of nanowires, nanotubes and nanodots. In this way, porous alumina can be fabricated electrochemically through anodic oxidation of aluminum, yielding highly ordered arrays of nano-holes several hundreds down to several tens of nanometers in size.
Sol-gel chemistry offers a flexible approach to obtain a diverse range of materials. It allows differing chemistries to be achieved as well as the ability to produce a wide range of nano-/micro-structures.
In bio-medical applications, sol-gel materials have been found to hold significant potential. One interesting application relates to hybrid materials that utilize sol-gel chemistry to achieve unusual composite properties. Another intriguing feature of sol-gels is the unusual morphologies that are achievable at the micro, and nano-scale. The ability to control pore chemistry at a number of different scales and geometries has proven to be a fruitful area of study, providing excellent bioactivity, and producing cellular responses and enabling the entrapment of biologically active molecules and their controllable release for therapeutic action.
Key words: Nanostructures, porous alumina AAO membranes, nanoporous anodic alumina (NAA), nanofabrication, sol-gel chemistry, nanocarriers, biomolecules.
1. Introduction
Porous alumina films formed by anodic oxidation of aluminum have been extensively studied for use as molds to form nanostructured materials. The technology of porous alumina and its usage as an anodic oxide coating in tools has a long history.
There is a great demand for the use of highly ordered nano-hole arrays, which can be produced on a scale of several tens of nanometers through self-organization, in a diversity of applications. These include high density storage media, functional nanomaterials exhibiting a quantum size effect, highly sensitive chemical sensors, nano-electronic devices and functional bio-chemical membranes.
Porous alumina membranes of anodic aluminum oxide (AAO) are widely used for the fabrication of various nanostructures and nano-devices. Over the last decade, many materials including nanowires, nanotubes and nanodot arrays, have been fabricated by the deposition of various metals, semiconductors, oxides and polymers inside the pores of AAO membranes (Fig. 1).
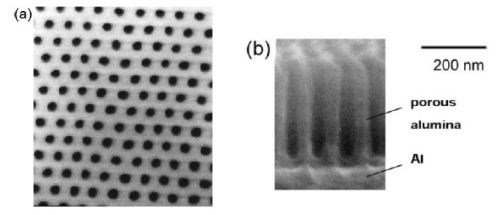
Figure 1 - SEM micrographs of an alumina nano-hole array formed by two-step anodic oxidation at 40 V using 0.15M oxalic acid: (a) plan-view, (b) cross-sectional view (Shingubara, et al., 1997).
Nanoporous substrates such as porous silicon, nano-porous anodic alumina, titania nanotube arrays and track-etched porous polymer membranes have been commonly employed as substrates for advanced sensing devices. Nanoporous anodic alumina (NAA) processes produce unique structural, chemical, optical, thermal and mechanical properties and biocompatibility in addition to controllable geometry and exploitable surface chemistries.
Ordered AAO stands out due to its remarkable properties such as chemical, thermal stability, hardness and high surface area. Over the past decade, we have witnessed the emergence of various applications based on AAO membranes such as molecular separation, chemical-biological sensing devices, cell adhesion, catalysis, energy storage and drug delivery vehicles (Fig. 2).
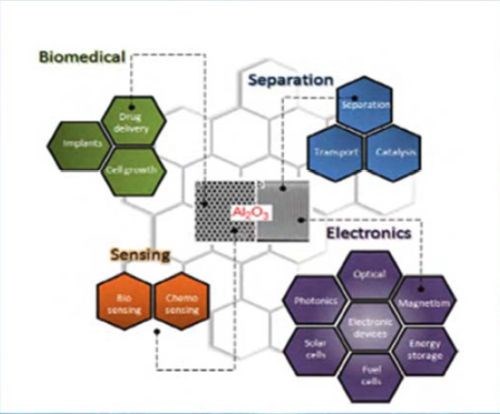
Figure 2 - Schematic diagram showing the typical AAO structure and the major applications for this nanostructured material.
Recent advances in fabrication procedures toward structural modifications and the generation of AAO structures with complex pore geometries, including branched, multilayered, modulated and hierarchically complex pores architectures are presented in Section 2.
Silica and doped silica materials obtained via solution gelation, or “sol-gel”, inorganic polymerization processes are also highly functional materials with an impressive range of applications, and utilize two of the pillars of chemistry: synthesis and analysis. Simple silica microspheres have since seen numerous applications, and today, the benefits provided by solution gelation are well recognized. In addition, advanced processing routes have also been developed that account for the problematic aspects of the gelation process.
Section 3 aims to describe the sol-gel synthesis routes that are most commonly used to produce ceramic and glass networks for biomedical applications. Sol-gel chemistry offers a flexible approach to obtaining a diverse range of materials. It allows different chemistries to be achieved and offers the ability to produce a wide range of nano-/micro-structures. In addition to providing an overview of the polymerization processes, the use of classical inorganic synthesis routes and colloidal aggregation will be discussed along with adaptations to the synthesis procedures that have allowed for further applications. Common links between methodologies are emphasized and the techniques themselves are discussed through recent applications.
Following this is a more detailed description of the biomedical areas where sol-gel materials have been explored and found to hold significant potential. One of the interesting fields that has been developed recently relates to hybrid materials that utilize sol-gel chemistry to achieve unusual composite properties. Another intriguing feature of sol-gels is the unusual morphologies that are achievable at the micro- and nano-scales.
2. Nanofabrication using a porous alumina template
Self-organized porous alumina nano-hole arrays have been used to fabricate a variety of nanomaterials. These methods are categorized as follows: etching of the semiconductor substrate using a porous alumina film as a mask, pattern transfer using porous alumina as a template for deposition of functional materials in the form of porous alumina nano-hole arrays by electroplating and sol-gel, and deposition of functional materials by chemical vapor deposition (CVD).
a. Porous alumina as an etching mask
The transfer of nano-holes to a semiconductor substrate is promising for applications such as photonic band materials, field emitter arrays and quantum dot arrays. Referring back to Fig. 1, a thin porous alumina film was used as a dry etching mask, by placing it in contact with the substrate. The porous alumina film was delaminated from the aluminum plate by a negative voltage pulse or dissolution of aluminum by dipping in HgCl2 solution. After removal of the nano-hole bottom barrier layer by argon plasma etching or ion beam etching, the porous alumina film was placed on the substrate.
Highly directional ion beam etching is necessary for substrate etching, since the alumina nano-hole aspect ratio (the ratio of depth to diameter ) is very high. The alumina mask showed high tolerance to Reactive Ion Beam Etching (RIBE) using a Br2/N2 mixed gas system (Shingubara, J. Nanoparticle Research, 5, 17-30 (2003)). In this method, maintaining the gap between the porous alumina and the substrate at a mínimum is essential for achieving ultrahigh uniformity.
Recently, an alternative method, using a porous alumina film deposited directly on the semiconductor substrate, was proposed by Shingubara (2003). A thin porous alumina film with an aspect ratio below 5 was formed on a Si/SiO2 substrate by the use of sputtered aluminum. Reactive ion etching using chlorine with a high self-bias of RF plasma proved effective for pattern transfer to Si. There was a significant reduction in hole size due to redeposition of nonvolatile materials on the side wall of the nano-holes. For instance, the initial porous alumina hole size of 40 nm was reduced to 10 nm Si holes when a higher aspect ratio of porous alumina nanoholes in the mask was used. The problem with this method was the non-uniformity of the porous alumina mask thickness, which would require a specially designed anodic oxidation electrode to improve the result.
b. CVD deposition on porous alumina
Chemical vapor deposition of materials in porous alumina nano-holes is a challenging topic for CVD research. Since porous alumina can contain extremely high aspect ratio holes, it is of great interest to discover how high aspect ratio holes can be filled by CVD. Working in a supercritical fluid medium is one way to obtain excellent disposition profiles.
Radium films were synthesized at controlled depths within porous alumina disks by the hydrogen reduction of organoradium compounds dissolved in supercritical CO2 at 50°C. Guided by a simple mass transport model, radium films ranging from 1 to 60 microns in thickness were deposited at prescribed depths between 60 and 500 microns.
The formation of carbon nanotubes (CNT) in porous alumina by CVD has been intensively studied. It is well known that CNT-CVD needs catalysis for thermal decomposition. A well ordered array, using electrodeposited Co and Nb located beneath the aluminum layer is shown in the SEM micrograph of Fig. 3.
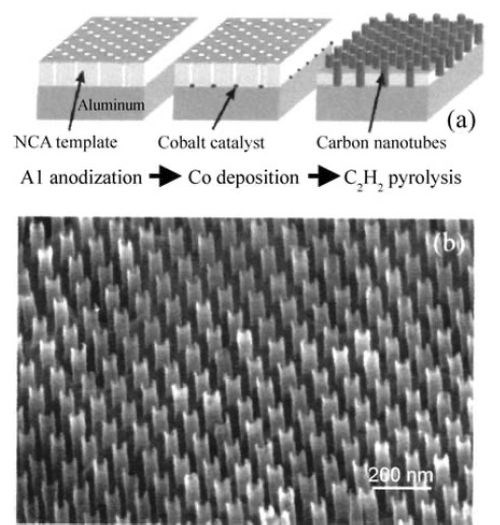
Figure 3 - SEM image of an array of carbon nano-tubes fabricated in a porous alumina template (Li, et al., 1999).
In the example shown in Fig. 3, using cobalt catalysis, pyrolysis of C2H2 was carried out at 600°C. Carbon nanotubes with diameters ranging from 10 to several hundred nanometers and lengths of up to 100 microns can be produced. This structure is highly promising for an ultrahigh-density field emitter array. CNT formed through Co catalysis by this method has a multi-walled structure. Low temperature deposition of CNT at around 500°C by microwave plasma-assisted CVD has also been reported.
c. Electroplating on porous alumina
Numerous studies have been conducted on the filling of conductive materials in porous alumina nano-holes by electroplating. Prior to electroplating, the bottom barrier layer should be thinned to less than about 15 nm. Wet chemical etching of the anodic alumina film using dilute chromic acid solution (pore widening treatment), or step-wise lowering of the anodic voltage to 15 V have been employed. Alternating current (AC) or pulsed current electroplating has been used since the impedance of the barrier layer at the nano-hole bottom is too large to allow for direct current (DC) electroplating. Research activity on electroplating magnetic materials in porous alumina has intensified remarkably in recent years. As for other metals, nanowire array formation of gold and silver have been reported.
3. Sol‐gel synthesis on porous alumina
Sol-gel provides an alternative synthesis route for nanomaterial fillings in porous alumina nano-holes.
Monodispersed hollow nanocylinders containing crystalline titania particles have been filled by an aqueous solution of titanium tetrafluoride.
Hollow nanotubes comprised of In2O3 and Ga2O3, have been synthesized by sol-gel chemistry and sol-gel synthesis of an array of C-70 single cristal nanowires in a porous alumina template.
a. Organic precursors in sol-gel methods
Silicon alkoxides represent the main network forming agents used in sol-gel preparation methods. While the sol-gel process provides key benefits, such as the low synthesis temperatures and the vast array of alkoxide precursors available, the cost associated with alkoxide precursors presents some limitations.
Nevertheless,the efficiency provided by low temperature synthesis and the accuracy with which specific compositions can be achieved have the potential to outweigh any such negative aspects of the process. Low temperature synthesis is achieved through solution-mediated formation of strong covalent bonds between elements that would otherwise require excessively high temperatures to create. For alkoxides this requires initial hydrolysis of the alkoxy group followed by as condensation between network forming substrates (Fig. 4).
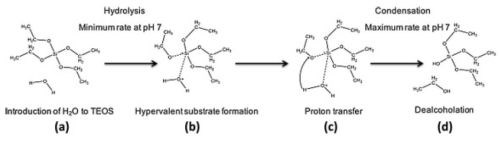
Figure 4 - Initial hydrolysis and condensation stages of tetraethyl orthosilicate - Si(OC2H5)4 (TEOS) in the production of silica oligomers: (a) Introduction of water to TEOS, (b) H2O forms a hypervalent substrate with silicon, (c) transfer of the proton from the water to the adjacent alkoxy group, (d) cleavage of the ester bond and dealcoholation.
Sol-gel methods also enable the powderless processing of glasses, ceramics and thin films or fibers directly from solution. Precursors are mixed at the molecular level and variously shaped materials may be formed at much lower temperatures than is possible by traditional preparation methods.
One of the major advantages of sol-gel processing is the possibility of synthesizing hybrid organic-inorganic materials. Combinations of inorganic and organic networks facilitate the design of new engineering materials with diverse properties for a wide range of applications. Biomedical applications invariably require the design of new biomaterials, and this can be achieved by emerging sol-gel chemistry and biochemistry. The gel-derived materials are excellent model systems for studying and controlling biochemical interactions within constrained matrices with enhanced bioactivity because of their surface chemistry, micro-/nano-pores and large specific surface area. In biomedical applications, the coating of medical devices is an important issue. Materials used in medical devices should have appropriate structural and mechanical properties and ideally promote a healing response without causing adverse immune reactions. Medical services designers currently use various surface treatments such as coatings that enhance or modify properties such as lubricity, the degree of hydrophobicity, functionalisation and biocompatibility. Sol-gel technology offers an alternative technique for producing bioactive surfaces for these applications.
Sol-gel thin film processing offers a number of advantages including low-temperature processing, ease of fabrication, and precise microstructural and chemical control. The sol-gel derived film or layer not only provides a good degree of biocompatibility, but also a high specific surface area and an external surface whose rich chemistry allows ease of functionalization by suitable biomolecules.
The development of multifunctional nanoparticles that can be used as drug delivery vectors remains a significant challenge of material science. These applications require intimate control of the particle size and discrete, superparamagnetic iron oxide nanoparticles that can be prepared by the sol-gel method to lower the annealing temperatures required.
Silica-based magnetic nanocomposites, formed by magnetic nanoparticles (MNP) dispersed in a silica matrix, are of relevant technological and scientific interest. Here encapsulation in silica prevents interactions between the MNPs, and consequently assures a uniform dispersion. The latter is essential for efficient performance in most applications, including the diagnostic and therapeutic areas, where particles must display high magnetization, be stable against oxidation and most importantly, remain non–aggregated.
Engineering new bone tissue with cells and a synthetic extracellular matrix (ECM) represents a promising approach for the regeneration of mineralized tissues. Bone regeneration requires a scaffold material upon which cells can attach proliferate and differentiate into functionally and structurally appropriate tissues for the body location into which they are placed .
Bone is a highly mineralized tissue consisting of an apatitic mineral phase most similar to a form of carbonated hydroxyapatite (HCA), although a significant contribution is made by extraneous ions such as sodium chloride, zinc and, to a lesser extent, fluorides. In general, HCA can be considered a model mineral for natural bone and is widely accepted as a bioactive material with excellent biocompatibility, high osteoconductivity, and reasonable mechanical strength. For these reasons, it has been widely used in tissue engineering applications, especially for bone and cartilage regeneration.
However, in vivo data suggest that degradation or ion release from labile sources such as bioactive glasses promotes new bone formation, as opposed to the relatively lower ion release that occurs as a result of HCA minerals reaching equilibrium with their surrounding medium. It appears that sol-gel methods hold the potential to apply an ever-increasing range of glass-based bioactive coating to materials, which have previously remained incompatible with alternative coating techniques. Furthermore, the versatility of the sol-gel approach is opening new doors to previously unattainable compositions, again increasing the potential applications of sol-gel materials as fillers to replace tissue within necrotic or defect sites.
Sol-gel microencapsulation technology and its broad application potential are now well-known. Relevant here is the use of silica for encapsulation and controlled release of both hydrofilic and hydrophobic molecules, ensuring considerable chemical and physical protection of the valued entrapped dopants. Given the above, it can be seen that sol-gel derived bioactive materials hold great potential value.
b. Sol-gel methods and reactions processes
The sol is a colloidal suspension of solid particles, whereas a gel is an interconnected network of solid-phase particles that form a continuous entity throughout a secondary, usually liquid phase. Throughout sol-gel technology, these phases are conserved though the chemical reactions that take place during the gel evolutions, and can be manipulated in a variety of ways, e.g., altering the initial precursors, time allowed for gelation, catalysts, degree of solvation, gelation conditions or physical processing of the gel itself. Sol-gel processes allow the formation of solid materials through gelation of solutions and can be used to produce a large number of useful morphologies. The processes are illustrated in Fig. 5.
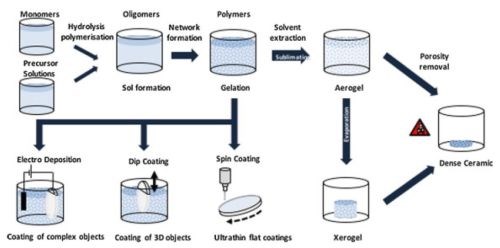
Figure 5 - Sol-gel synthesis routes: Processes are defined as sol-gel by the transition of a colloidal solution to an interconnected gel network (gelation). The further processing stages illustrated are non-redundant and may be combined depending on the specific needs of the application.
What remains constant for the production of sol-gel derived bioactive materials are the stages that allow for hydrolysis and condensation reactions to occur. Successful manipulations of these reactions are shown in Fig. 6.
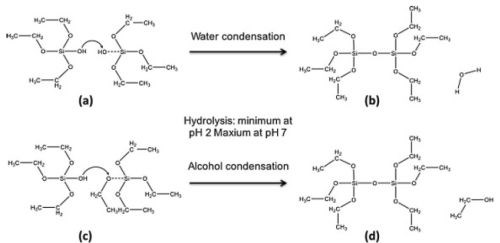
Figure 6 - Subsequent condensation stages of TEOS in the production of silica oligomers. Condensation between silanol groups on two hydrolyzed TEOS molecules (a and b) and between a silanol group and an adjacent alkoxy group (c and d) result in the production of free H2O and ethanol respectively.
Figure 6 shows that only one reaction occurs during condensation: the loss of an HO-group from the substrate. This mechanism is therefore a reaction that can occur as either dehydration or dealcoholation. For the former to occur, two HO-groups must take part in the formation of an Si-O-Si bond, whereas the latter results from the direct transfer of a proton to the leaving group on a neighboring substrate.
As evident from these reactions, a decrease in pH can promote hydrolysis through protonation of the leaving groups. Alternatively, higher pH will induce the deprotonation of OH- groups and therefore favor condensation. However, OH- is a highly efficient nucleophilic species and electron transfer from -OH groups can be facilitated by H+ in the immediate environment. This relationship means that higher and lower pH values are also able to promote condensation and hydrolysis, respectively. In silica-based systems, the reactions proceed by acidic catalysis at pH < 2.5 and basic catalysis when pH < 2.5 ,which can be explained by the isoelectric point of silica at pH 2.5.
In the sol-gel route synthesis, a stepwise reaction scheme has been undertaken to control the ratio of hydrolysis to condensation rates. In general, the rate of hydrolysis is fast compared to that of condensation in strong acidic conditions. Therefore, a well-ordered hexagonal arrangement of mesopores (a pore structure that is commonly formed in sol-gel silica materials) is formed at low pH in acidic conditions. Meanwhile, in neutral or basic conditions ranging from pH 7 to pH 9, the rate of condensation is faster than that of hydrolysis, and eventually the materials prepared by a single-step reaction at high pH display a gel-like structure often without mesopores. However, the higher electronegativity of transition metal species as compared to silicon and phosphorus can cause issues where condensation reactions proceed with an unfavorable bias away from the desired network composition or the end particulate structure.
Like silicon, phosphorus and vanadium precursors can be used as network-formers within the sol-gel process. This difference in network connectivity results in a more relaxed network structure when compared to silica-based networks, as silicate is able to share all four oxygen atoms with neighboring cationic groups. This in turn increases the range and quantity of species that are able to be included, but such flexibility comes at the expense of stability, as the high electronegativity of the =O bond leaves the network open to hydrolysis. As with conventional melt-derived materials, solubility can be controlled by the combination of network modifiers, as can the release of active agents or tailoring of other physical properties of the material in question.
The solvent itself also plays an important role in determining the rate of gelation reactions. This solvation effect can occur in two ways: through viscosity or hydration effects. However, the solvent is shown to alter the NiO2 crystalline structure, which serves to highlight the need for experimental confirmation as, with such a variety of avenues available to exploit, comes as set of variables that must also be controlled, including the effects of viscosity, or more precisely the dielectric constant.
By altering the solvent species from that of the alkoxide itself, the substrate can become coordinated with a mixture of alkoxy groups. Undoubtedly, this would affect polymerization in a way that is dependent on a specific combination of coordinated groups present on network-forming substrates throughout the solution.
The initial Si-O-Si bridges that are formed can be further strengthened by passive deposition of silica on the initial bridge as a result of the equilibrium orthosilicic acid Si(OH)4, and the silica making up the mass of the colloids. Furthermore, colloidal sol-gel methods are not limited to silica or silica-based systems.
The applicability of the colloidal methods is based on two key aspects of the process stabilization of the colloidal particles within the sol and coalescence or flocculation to form the gel. With particles that possess the same electrostatic charge, colloidal suspensions are maintained by the potential, which in turn reflects the magnitude of the electrical double layer present on charged particles in solution. As noted above, the removal of the solvent is one method by which the aggegation of colloidal particles can be achieved. Alternatively, altering the pH, salinity or temperature can induce depeptization, whereby the electrical double layer is reduced to a point where the potential is no longer strong enough to prevent attractive Van der Waals forces and flocculation takes place.
Despite the potential of colloidal methods to provide much thicker, more structurally resistant films and deposits than methods that rely on de novo synthesis,** this route has seen the least number of applications within biomedical research. The gelation rate correlates well with the structural stability of the encapsulated proteins and serves to demonstrate an ability to circumvent conditions that would otherwise damage sensitive molecules. For certain applications however, the colloidal sol-gel method does not provide the degree of protection required.
Colloidal methods offer a further benefit over alkoxide based systems in that the majority of the network is already present in the sol. Adaptations such as the introduction of osmoprotectants can therefore be applied without significantly interfering with the integrity of inorganic capsule itself.
c. Metal chelation in the sol-gel
In aqueous solution, metal ions are coordinated by a hydration shell, the nature of which depends both on the valence of the specific metal in question and the pH of the solution. This ultimately results in the formation of polymeric oxides in solution.
The hydroxy-ligand thus formed is able to act as a bridge between the two hydrated metal complexes. This results in the release of a proton into the aqueous medium and is followed by a subsequent deprotonation event resulting in a M-O-M covalent bond. From this brief description alone, the influence of pH on the process can also be deduced. An increase in pH favoring olation and the lower pH inhibiting the process.
Undoubtedly, materials composed of metal oxides exhibit a wide range of desirable properties and as a result, a series of methods have been developed based on chelation of metal precursors in order to control the natural polymerization processes. Essentially, metal chelation sol-gel methods employ strong chelating agents (such as citric acid or EDTA) as a means of controlling the formation of the highly reactive hydrated complexes.
Although discussed here in terms of chelated inorganic precursors, chelation itself is not limited to inorganic processes. Such methods can also be applied to modify the polycondensation of metal alkoxides whereby the rate of reaction is reduced following the replacement of alkoxide, leaving groups with a chelating ligand in more stable conformation.
In further discerning metal chelation methods from the alkoxide sol-gel route, the underlying principle is that polycondensation of the metal itself occurs through hydration processes described above, as opposed to the hydrolysis and condensation steps akin to the polymerization of organometallic precursors.
Based on a system that made use of triethanolamine as an Fe(II) chelating agent, triethanolamine is able to form chelation complexes with a wide range of transition metal elements, therefore offering a plausible route for further biologically relevant substitutions within the network.
The use of epoxides as gelation agents provides another useful synthesis pathway. Typically, epoxide routes are most effective when the formal oxidation state of the dopant cation is M+3, although species that possess a lower valence may also be incorporated. In this instance, the epoxide does not act as a precursor per se. Rather, the epoxide group is able to efficiently accept protons, leading to the formation of hydrated oxo-ligands M(H2O)n(O)m-n)+2 coordination until deprotonation in the presence of an epoxide.
Although not strictly a chelation-base methods parallels can be observed between this approach and the more typical chelation methods that aim to prevent natural polymerization processes until required.
d. Polymer assisted sol-gel
In a natural extension from metal chelates methods are the polymeric sol-gel methods. Essentially these methods involved the chelation of reactive inorganic gel-forming agents within an organic polymer network although, depending on the material to be produced, chelation is secondary to stabilization. In broader terms, gel forming agents are maintained within in a state of dispersion throughout the solution, thereby preventing the precipitation of aggregates within the sol. This method does however require subsequent heat treatment to remove the organic polymer following the formation of the inorganic gel.
Chemical properties,such as the biomimetic*** molar ratios of apatites, can also be achieved with polymer assisted stabilization due to the homogeneous elemental distribution of the gel network.
Inorganic networks can also be formed in situ through the polymerization of organic precursors. This method involves the formation of a three-dimensional polyester network resulting from the reaction between ethylene glycol and citric acid. The citric acid acts as a chelation agent due to an available bi-dentate binding mechanism followed by an esterification with ethylene glycol (Fig. 7). The organic network would be removed as with ex situ polymers as described above.
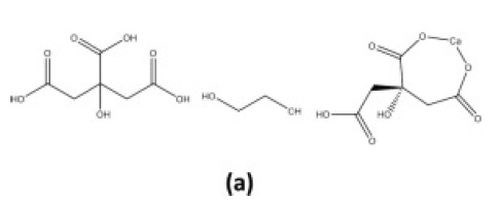
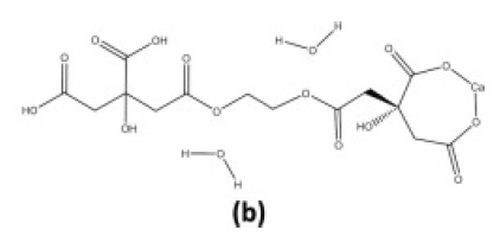
Figure 7 - Esterification (condensation) of ethylene glycol and citrate in the presence of a cationic ligand (calcium).
Effective production of both biocompatible ceramic-mineral composites and apatites with predefined stoichiometry has been achieved, with polymer-assisted sol-gel methods. Such research may also have broader implications than in solving issues associated with biocompatibility as, with the advent of controlled deposition of inorganic mineral layers, a biotic hard tissue regeneration may also be within reach.
e. Silica-based sol-gel materials
Silica-based sol-gel materials have been the subject of intense interest for the last three decades. Biomolecular encapsulation within sol-gel-derived silica matrices was first successfully achieved by entrapping enzymes into TEOS matrices.
During the last few decades, silica-based materials have supplied successful solutions for soft and hard tissue regeneration. These materials are highly biocompatible and the positive biological effects of their reaction products make them an interesting group of materials for tissue regeneration. Silica-based bio-reactive glasses were first synthesized via a sol-gel technique at lower processing temperatures compared to the melt derived glasses. Extensively researched sol-gel glasses were based on the SiO2-ONa-P2O3 system for biomedical apllications. Silica-based sol-gel glasses exhibit many of the properties associated with an ideal material for tissue regeneration, such as high surface area and a porous structure, in terms of overall porosity and pore size, that promote cell-material interactions and cell invasion. Research on these glasses showed that their porous structure exhibits a higher surface area that exhibits higher tissue bonding rates.
A sol-gel process, involving the foaming of a sol with the aid of a surfactant, followed by condensation and gelation reactions, has been used to prepare porous scaffolds of a few bioglasses, such as the glass designated with the composition (mol%): 40 SiO2-2O-ONa-2P2O3. The as-prepared scaffold had an overall microstructure similar to that of dry human trabecular bone, but the pore structure was hierarchical consisting of interconnected macropores <90 microns, resulting from the forming process and mesopores that are inherent to the sol-gel process.
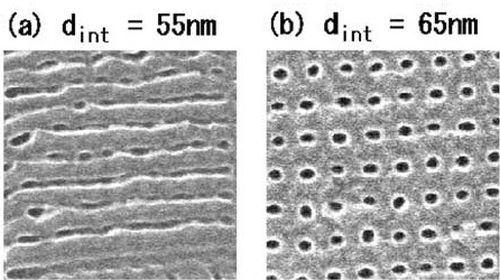
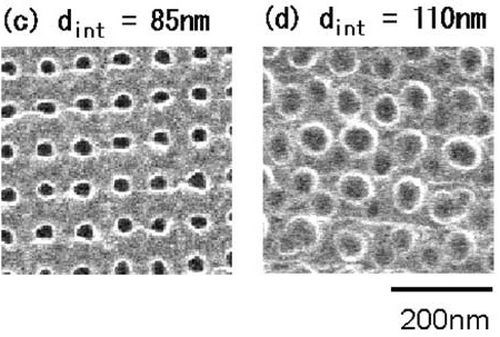
Figure 8 - Plan-view SEM micrographs of porous alumina film surfaces that were formed by AFM nano-indentation, followed by anodic oxidation at 40 V using 0.15M oxalic acid for 5 min. Indentation force was 4.16×10−5 N. The indentation interval was varied from 55 to 110 nm. (Shingubara, et al., 2002).
Figure 8 illustrates the porous structure of the scaffolds made of bio-active glasses produced by means of the sol-gel processes. This hierarchical pore structure of the scaffolds is beneficial for stimulating interaction with cells as it mimics the hierarchical structure of many natural tissues and more closely simulates the physiological environment of mineralized tissues. Thanks to the nanopores in the glass, sol-gel derived scaffolds have a very high surface area (100-150 m2/g). As a result, these scaffolds degrade and convert faster to via a hard anodizing process than those of melt-derived glass with the same composition. However, these sol-gel-derived scaffolds have a relatively low compressive strength, and consequently, they are primarily suitable for applications focused on low load bearing orthopedic sites.
The discovery of mesoporous silica nanoparticles was quickly recognized as a breakthrough that could lead to a variety of important applications. These materials have uniform cylindrical pores with a diameter size range of 2 to 20nm, along with a large surface area between 500-1100 m2/g, making them ideal for chemical separation, catalysis and biomedical applications. In general, solution syntheses were carried out under a basic condition similar to the traditional method for preparation of silica particles.
Under basic conditions, a part of the silanol group Si-OH, is deprotonated to form silanolate, Si-O. In order to match the negatively-charged silica, cationic surfactants are normally used. Thus, the hydrolysis and condensation rates of the silica sources are highly associated with several factors such as pH, silica source, additives and temperature. The effect of pH is critical as it affects nucleation and growth. Previous reports hint that a minimum particle size can be obtained at around pH 9-10, indicating that the condensation rates, rather than the hydrolysis rate of silica precursors, highly affect the final particle sizes.
There have been few trials for preparing sol-gels under acidic conditions. One of the advantages of using acidic conditions is the capability of using block copolymers,which have a templating effect and allow larger mesopores, 5 nm. The condensation rate for the synthesis at pH 5-8 should be faster than at the lower pH of 5. Since the condensation reaction solidifies the silica network, one can expect that a too rapid condensation reaction will create a cross-linked network faster than the mesostructure is organized.
This explanation is based on a formation mechanism where the particle formation, silica condensation, and ordering of the pore system are considered as separate processes. Thus, in order to obtain an ordered material, the rates of the different processes must be properly adjusted relative to each other. Compared with basic synthetic systems however, it is difficult to obtain a uniform particle size less than 90 nm, and with spherical morphologies, the large surface area of the pores allows them to be filled with different biomolecules for biomedical applications.
4. Summary
This work is much more extensive than what is covered here, and includes the following areas of development:
- Organic and inorganic hybrid materials
- Chemical and inorganic hybrid materials
- Chemical and biological properties
- Morphology and shape
- Interaction with cells and intracellular delivery
- Biomedical applications
- Sol-gel bioactive glasses and hybrids
- Nano-carriers to deliver bio-molecules
- Multifunctional sol-gel nano-carriers to deliver bio-molecules
- Multifunctional sol-gel nano-carriers for diagnostic purposes and
- Sol-gel based sensing devices.
As with hard anodizing and nanotechnology with nano-structures and pores, sol-gel technology is increasingly being integrated into the technology of the pharmaceutical, biological and food industries, etc., with surface treatments and proper metal selection for different applications.
Over the past several years, significant progress has been made with regard to structural engineering and surface modification of nanoporous AAO material. Much of this progress has been application-driven. In this review, we have drawn together innovative approaches on controlling and designing the structural growth of AAO with different sizes, arrangements, structures, geometries and pore architectures. Sol-gel synthesis offers a molecular-level mixing and is capable of improving the chemical homogeneity of the resulting composite.
5. Conclusions
Sol-gel derived nanostructured material is applicable to biomedical-separation-sending and electronics. Compatibility is but one facet of sol-gel derived biomedical applications.
Sol-gel derived bioceramics have a great potential for application as a coating on metallic substrates, providing a high degree of biocompatibility and promoting a rapid heating response with minimal averse biological events.
An electrically-responsive electro-polymer-coated AAO membrane shows reversible change of pore size between oxidation and reduction states.
Silanization with AAO silane multilayers is described.
Since sol-gel processes are carried out at such low temperatures, they can also allow the inclusion of biomolecules, and therapeutic agents, including drugs, growth factors and proteins. These can be loaded during the sol-gel preparation process and released in a controlled manner.
The sol-gel method should be simple stable, cost-effective and scalable for facilitating future industrial production and clinical translocation.
Polyelectrolytes of porous AAO membranes can be modified by adsorption of two polyelectrolytes coupling of an antibody.
Researchers are pushing the boundaries of molecular separations using AAO with pores of controlled shape and size, internal surface modification and are exploring the effects of external parameters, such as pH, flux, concentration gradient and ionic strength.
Fabrication of complex AAO nanostructures combined with even greater control over surface functionality is expected to lead to unique nanostructures and nano-devices with unprecedented functional properties for the next generation devices including the exploration of their application with a focus on a different research areas ranging from medicine to material science and electronics.
6. Bibliography
1. T. Kondo, H. Masuda and K. Nishio, “SERS in Ordered Array of Geometrically Controlled Nanodots Obtained using Anodic Porous Alumina,” J. Phys. Chem., C117 (6), 2531-2534 (2013).
2. J.M. Rosenholm, C. Sahigren and M.T. Lindén, “Towards Multifunctional, Targeted Drug Delivery Systems using Mesoporous Silica Nanoparticles - Opportunities & Challenges,“ Nanoscale, 2 (10), 1870-1883 (2010).
3. T. Kumeria, L. Parkinson and D. Losic, “Bioinspired Microchip Nano-porous Interferometric Sensor for Sensing and Biosensing Applications, “Micro & Nanosystems,” 3 (4), 290-295 (2011).
4. P. Yang, S. Gai and J. Lin, “Functionalized Mesoporous Silica Materials for Controlled Drug Delivery,” Chem. Soc. Rev. 41 (9), 3679-3698 (2012).
5. D. Avnir, “Organic Chemistry within Ceramic Matrixes: Doped Sol-gel Materials,” Acc. Chem. Res., 28 (8), 328-334 (1995).
6. R. Ciriminna, et al., “The Sol-gel Route to Advanced Silica-based Materials and Recent Applications,” Chem. Rev., 113 (8), 6592-6620 (2013).
7. R. Dronov, et al., “Nano-porous Alumina-based Interferometric Transducers Ennobled,” Nanoscale, 3 (8), 3109-3114 (2011).
8. L.L. Hench, “Sol-gel Materials for Bioceramic Applications,” Curr. Opin. Solid State Mater. Sci., 2 (5), 604-610 (1997).
9. O.R. Hughes, in Ultrastructure Processing of Advanced Ceramics, J.D. MacKenzie and D.R. Ulrich , Eds., Wiley-Interscience, New York, 1988.
10. T.J. Graham, “On the Properties of Silicic Acid and Other Analogous Substances,” J. Am. Chem. Soc., 17, 318-327 (1864).
11. G. Jeon, et al., “Electrically Actuatable Smart Nano-porous Membrane for Pulsatile Drug Release,” Nano Lett., 11 (3),1284-1288 (2011).
12. J.D. Wright and N.A.J.M. Sommersjik, Sol-gel Materials: Chemistry and Applications, 1st Edition, CRC Press, Boca Raton, Florida, USA, 2000.
13. M. Ebelmen, “Recherches sur les combinaisons des acides borique et silicique avec lese thers,” Ann. Chim. Phys., 16, 129-166 (1846).
14. A. Balamurugan, S. Kannan and S. Rajeswari, “Structural and Electrochemical Behavior of Sol-gel Zirconia Films on 316L Stainless Steel in Simulated Body Fluid Environment,” Mater. Lett., 57 (26-27), 4202-4205 (2003).
15. D.B. Haddow, P.F. James and R. Van Noort, “Sol-gel Surfaces for Biomedical Applications,” J. Mater. Sci. Med., 7, 255-260 (1996).
16. S. Radin and P. Ducheyne, “Controlled Release of Vancomycin from Thin Sol-gel Films on Titanium Alloy Fracture Plate Materials, Biomaterials, 28 (9), 1721-1729 (2007).
17. D.E. Lee, et al., “Multifunctional Nanoparticles for Multimodal Imaging and Theragnosis,” Chem. Soc. Rev., 41 (7), 2656-2672 (2012).
18. A. Hervault and N.T.K. Thanh, “Magnetic Nanoparticle-based Therapeutic Agents for Thermo-chemotherapy Treatment of Cancer, Nanoscale, 6 (20), 11553-11573 (2014).
19. A.H. Lu, E.I. Salabas and F. Schuth, “Magnetic Nanoparticles: Synthesis, Protection, Functionalization and Applications,” Angew. Chem. Intl. Ed., 46 (8), 1222-1244 (2007).
20. A.Sen, et al., “Use of Nanoporous Alumina Surface for Desorption Electrospray Ionization Mass Spectrometry in Proteomic Analysis,” Biomed. Microdevices, 10 (4), 531-538 (2008).
21. K. DeGroot, “Bioceramics Consisting of Calcium Phosphate Salts, Biomaterials, 1 (1), 47-50 (1980).
22. D.M. Liu, et al “Structural Evolution of Sol-gel-derived Hydroxyapatite,” Biomaterials, 23 (7), 1679-1687 (2002).
23 R. Ravikrishna, M. Ren and K.T. Valsaraj, “Low-temperature Synthesis of Porous Hydroxyapatite Scaffolds using Polyaphron Templates,” J. Sol-Gel Sci. Technol., 38 (2), 203-210 (2006).
24. F. Foroutan, et al., “Novel Sol–gel Preparation of (P2O5)0.4–(CaO)0.25–(Na2O)X–(TiO2)(0.35−X) Bioresorbable Glasses (X = 0.05, 0.1, and 0.15),” J. Sol-Gel Technol., 73 (2), 434-442 (2015).
25. R. Ciriminna, et al., “From Molecules to Systems: Sol-gel Microencapsulation in Silica-based Materials,” Chem Rev., 111 (2), 765-789 (2011).
26. M. Vallet-Regi, “Ceramics for Medical Applications,” J. Chem. Soc.: Dalton Trans., 2 (2), 97-108 (2001)
27. W. Stober, A. Fink and E. Bohn, “Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range,” Colloid Interface Sci., 26 (1), 62-69 (1968).
28. Z.D. Hendren, J. Brant and M.R. Wiesner, “Surface Modification of Nanostructured Ceramic Membranes for Direct Contact Membrane Distillation,” J. Membrane Sci., 331 (1-2), 1-10 (2009).
29. E.K. Schmitt, C. Weichbrodt and C. Steinem, “Impedance Analysis of Gramicidin Din Pore-suspending Membranes,” Soft Matter, 5 (17), 3347-3353 (2009).
30. Q. He, et al., “Self-assembly of Composite Nanotubes and Their Applications,” Current Opin. Colloid Interface Sci., 14 (2),115-125 (2009).
31. R. Barbey, et al., “Polymer Brushes via Surface-initiated Controlled Radical Polymerization: Synthesis, Characterization, Properties and Applications,” Chem. Rev., 109 (11), 5437-5527 (2009).
32. M.A.C. Stuart, et al., “Emerging Applications of Stimuli-responsive Polymer Materials,” Nat. Mater., 9 (2), 101-113 (2010).
33. A. Zhang, et al., “Synthesis of Silica Nanotubes with Orientation Controlled Mesopores in Porous Membranes via Interfacial Growth,” Chem. of Mater., 24 (6), 1005-1010 (2012).
34. J.D. Berrigan, et al., “Protein-enabled Layer-by-layer Syntheses of Aligned, Porous-wall, High-aspect-ratio TiO2 Nanotube Arrays,” Adv. Funct. Mater., 21 (9), 1693-1700 (2011).
35. C. Marichy, M. Bechelany and N. Pinna, “Atomic Layer Deposition of Nanostructured Materials for Energy and Environmental Applications,” Adv. Mater., 24 (8), 1017-1032 (2012).
About the author

Dr. Xavier Albort Ventura earned his Ph.D. in Chemical Engineering Industrial from the University of Barcelona Spain. He is a member of the Barcelona International Trade Fair and has organizing responsibility for the Environment Technical Meetings and at the Eurosurfas show. He is an electroplating consultant for more of 50 European companies from various countries, including Portugal, France, Spain, Italy and Germany. Dr. Ventura has been an active presenter at SUR/FIN, having given papers since 1987. He has also been an active participant at Interfinish, presenting papers and conducting workshops since 1984. He has published more than 200 papers in Spain, the United Kingdom, France, the United States and Brazil, and has won a number of national and international awards in the United States. He received the Sam Wyman Memorial Award at SUR/FIN 2004 in Chicago for the Best Light Metals paper, “Sealing Method for Anodizing Aluminum and Hard Anodizing and a Test to Determine the Seal Quality of Aluminum.”
*Corresponding author
Xavier Albort Ventura, Professor and Consultant
University Politecnic of Barcelona Spain Research and Development
C. Carabela.la La Niña, 22-24 bis
Barcelona, Barcelona Spain 08017
Phone: 34-93-2054936
Mobile: 34-63-9721065; 34-60-1370304
E-mail: xavieralbort@ing-mediamb.com
**De novo synthesis refers to the synthesis of complex molecules from simple molecules.
*** Biomimetic refers to synthetic methods which mimic biochemical processes.
Related Content
Finishing Systems Provider Celebrates 150 Years, Looks to Future
From humble beginnings as an Indiana-based tin shop, Koch Finishing Systems has evolved into one of the most trusted finishing equipment providers in the industry.
Read MoreChicago-Based Anodizer Doubles Capacity, Enhancing Technology
Chicago Anodizing Company recently completed a major renovation, increasing its capacity for hardcoat anodizing and Type II anodizing.
Read MoreBryan Leiker, MFACA, Discusses CARB Public Hearing Over Calif. Hex Chrome Ban
Bryan Leiker, executive director, Metal Finishing Association of California, offers a recap of a January 27, 2023, public hearing conducted by the California Air Resources Board prior to an impending ruling on a proposed ban of hexavalent chromium use for finishing operations in the state.
Read More10 Anodizing Best Practices
Following this list of guidelines can help to increase the performance, cost effectiveness and quality for your anodizing operation.
Read MoreRead Next
A ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read More








.jpg;maxWidth=300;quality=90)











