Non-PFOS, Permanent Mist Suppressants for Hard Chromium Plating, Decorative Chromium Plating and Chromic Etch Applications
Mist suppressants are an important tool for reducing exposure of workers to Cr(VI) during plating and etching operations. A common raw material used for efficient mist suppressants was, and still is, PFOS. PFOS is subject to legislation across the world prohibiting its use for many applications. In this paper we will present developments and experiences with non-PFOS, permanent and non-permanent mist suppressants.
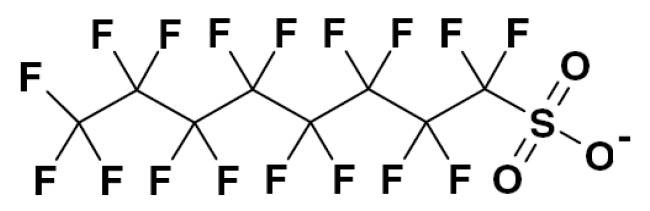
The negative health effects of PFOS on animals and humans have been studied and were found to be unacceptable.1,2 As a result, there are several legislations worldwide restricting the marketing, production and sales of substances, including PFOS. In the U.S., the EPA3 included PFOS in a new table of substances now subject to Significant New Use Rules (40 CFR 721.9852). This rule became effective on November 11, 2007. There is an exemption for one form of PFOS, with CAS number 56 773-42-3, when it is used for metal finishing and electroplating applications, including hard and decorative chromium plating, chromic acid anodizing or reverse etching, etching plastics prior to metallization and nickel plating. There is a more restrictive PFOS directive from the EU4 that came into effect on June 27, 2008. Further, there is a worldwide directive from the Stockholm Convention of Persistent Organic Pollutants, effective August 26, 2010, which affects over 150 countries including Japan, implementing an effective ban of sales and production of all PFOS-containing products starting April 1, 2010.
One of the main aims of these directives is ultimately to ensure the phase-out of the utilization of PFOS. There are currently exemptions in most of these directives for hard chromium plating.5 However, there are several communities, organizations and authorities that are campaigning for a complete ban on PFOS use.
Due to the increased awareness of the HES risks, the use of PFOS has declined by over 95% in the last decade, leaving only a few specialized applications. As the health and safety implications of the use of fume suppressants for Cr(VI) applications are serious, a suitable replacement for PFOS must be rigorously tested to ensure its suitability. Investigations into a large variety of alternative, non-PFOS candidate compounds have been going on for several years.
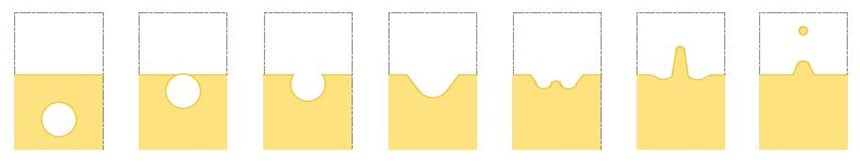
Typical criteria for a good mist suppressant are:
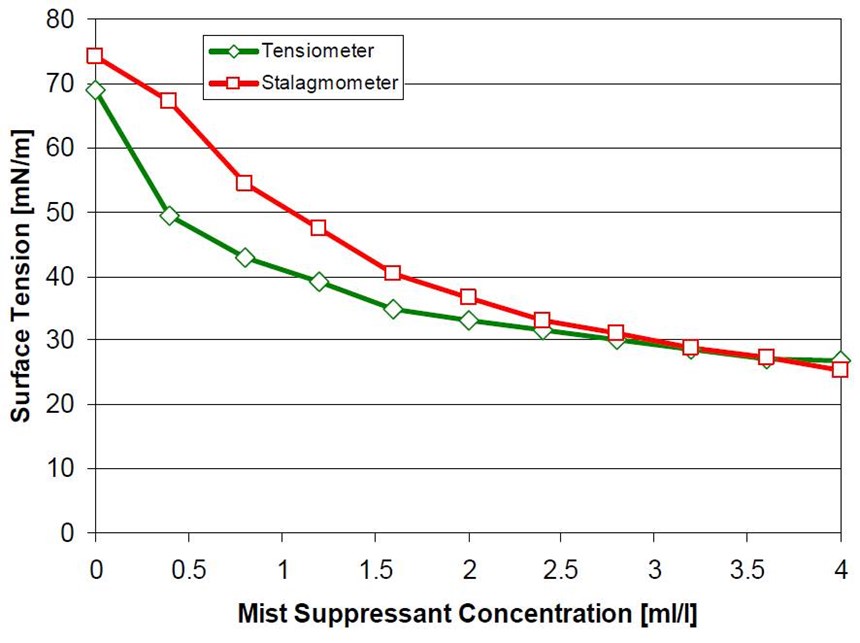
The surface tension of a chromium plating bath can be reduced to 20 dynes/cm using the non-PFOS products, but is commonly kept at an average of 30 dynes/cm. At this level, consumption of the mist suppressant is kept to a minimum and the Cr(VI) emissions are kept under control.
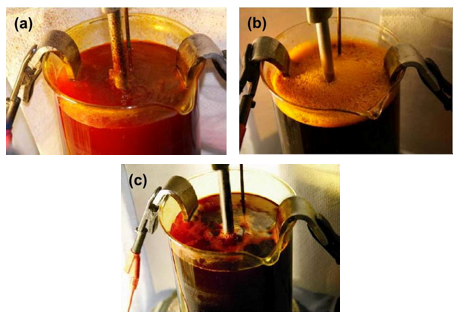
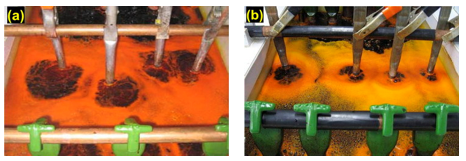
When changing from a PFOS-based product to a non-PFOS product, there are two options: a completely new make-up or a slide conversion. Both methods have been employed and studied. The most common method is to perform a slide conversion of an existing solution. This is generally an easy operation. The surface tension of the bath is analyzed and the required amount of non-PFOS product is then added to attain the desired surface tension. The non-PFOS product is subsequently dosed to maintain the surface tension within the desired range.
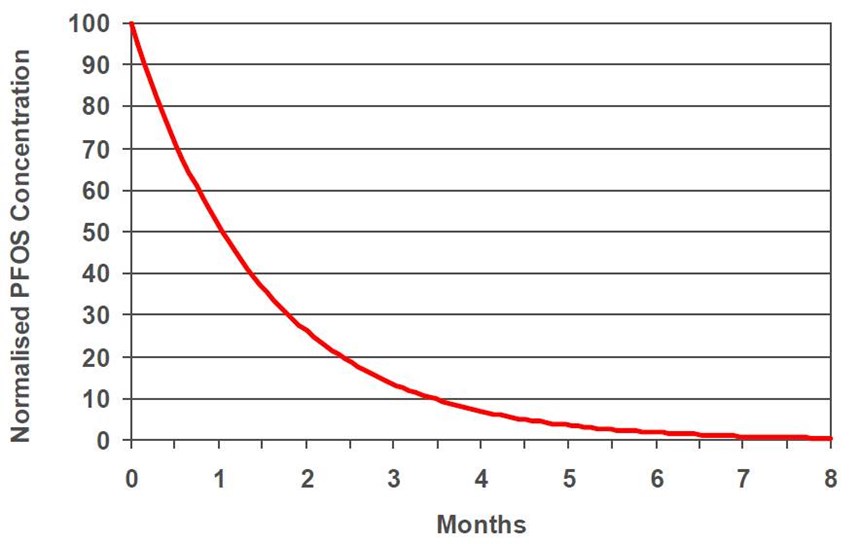
When a slide conversion is made, under normal circumstances, the PFOS concentration in the solution should reduce over time. One example of a slide conversion (Fig. 12) shows the potential decline in PFOS over time. This was a decorative chromium bath that was converted to a foaming type non-PFOS mist suppressant.
It took seven months to reduce the PFOS content to only 1% of the original level at this particular commercial plater. Different installations may yield different results due a number of factors.
In conclusion, there are now suitable, successful and well proven permanent non-PFOS mist suppressants available in the market, both foaming and low-foaming, for hard chromium and decorative chromium plating as well as chromic acid etching.
Related Content
Finishing Systems Provider Celebrates 150 Years, Looks to Future
From humble beginnings as an Indiana-based tin shop, Koch Finishing Systems has evolved into one of the most trusted finishing equipment providers in the industry.
Read MoreA Smooth Transition from One Anodizing Process to Another
Knowing when to switch from chromic acid anodizing to thin film sulfuric acid anodizing is important. Learn about why the change should be considered and the challenges in doing so.
Read MoreBryan Leiker, MFACA, Discusses CARB Public Hearing Over Calif. Hex Chrome Ban
Bryan Leiker, executive director, Metal Finishing Association of California, offers a recap of a January 27, 2023, public hearing conducted by the California Air Resources Board prior to an impending ruling on a proposed ban of hexavalent chromium use for finishing operations in the state.
Read MoreRead Next
A ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read More













.jpg;maxWidth=300;quality=90)












