Preventing Dark Streaks in Trivalent Chromium
Matt Schario from Columbia Chemical explains what is the likely cause of these streaks and patterns, and how to prevent this from happening.
Q: We plate aftermarket automotive pieces and have experienced dark streaks and patterns out of our trivalent chromium plating solution. What is the likely cause and how can we prevent this from happening?
A. Significant advancements have been made in trivalent chromium plating technology over the past decade with regard to improved color, resin technologies and understanding how to properly maintain solutions in production settings. With all of these improvements, however, it is still important to understand that trivalent chromium plating requires close monitoring with special attention to reducing contamination loads.
Dark patterns in trivalent chromium plating can be caused by the following:
- Metallic contamination
- Complexer imbalance
- Insufficient anti-mist additive
- Improper balance in proprietary salts
Metallic Contamination: Unlike hexavalent chromium solutions, trivalent chromium plating solutions are especially susceptible to metallic contamination. The most common types of metallic contamination are nickel, copper, zinc and iron. Nickel contamination is the most common as the nickel solution can drag down the plating line and find its way into the trivalent chromium solution. Copper and zinc contamination mainly come from brass substrates and zinc die cast where unplated surface area dissolved in the trivalent chromium plating solution due to low pH. Lastly, iron contamination can be looked into as a factor in causing dark deposits. To remove metallic contamination, it is critical to ensure the ion exchange is properly sized to the trivalent chromium solution and, most importantly, to utilize a high quality resin that has a strong affinity toward metals, such as copper, nickel and zinc. It is also necessary to regenerate the ion exchange resin regularly to ensure optimum effectiveness to continually pull metals out of the solution. Additionally, some suppliers have modern technology mist suppressants that not only lower surface tension but also provide high tolerance to metallic impurities.
Complexer Imbalance: Chrome that is not fully complexed will not bind and will cause dark patterns, so it is necessary for trivalent chromium solutions to contain a complexer that enables the chromium to plate correctly. It is important to ensure the solution contains the correct amount of complexer and, finally, that the chromium in solution is fully complexed. This can be achieved by heating the solution to 140°F for 4-8 hours and then cooling it to the recommended operating temperature.
Insufficient Anti-Mist Additive: Anti-mist additives are designed to lower the surface tension of the working solution and improve deposit distribution. Modern (newer technology) anti-mist additives also provide additional resistance to metallic contamination. The graphs in Figure 1 show the comparison of deposit distribution through the use of older technology anti-mist versus modern technology anti-mist additive. The anti-mist additives are generally consumed in very small amounts and can be analyzed using a tensiometer or any other means to check surface tension in the solution. Please refer to your supplier’s recommendations for specific anti-mist ranges as these components can vary from supplier to supplier. When the anti-mist additive is not sufficiently maintained or relies on older technology, the deposit distribution can be affected, leading to a darker deposit or dark streaks.
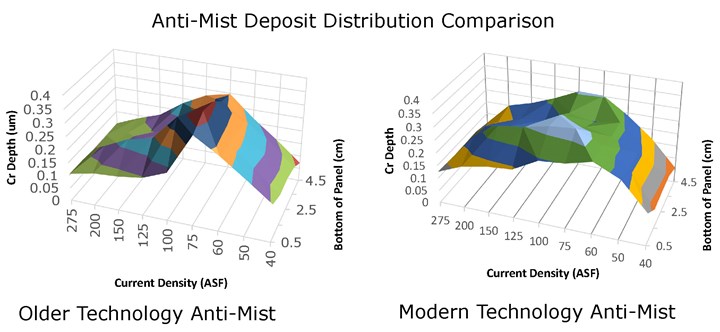
The comparison of deposit distribution through the use of older technology anti-mist versus modern technology anti-mist additive.
Improper Proprietary Salt Balance: A final cause behind dark streaks or deposits is the blend of proprietary salts. This is often the more challenging factor because it relies upon a trusted relationship between the customer and a knowledgeable chemistry supplier. These proprietary salts are designed to provide the necessary conductivity of the solution. The proprietary salt blend also contains additives that improve high-current density buffering and specialty additives that prevent the chromium from converting from trivalent to hexavalent. It’s important to make sure the proprietary salts are operating at or near 100% of the recommended parameters at all times, in order to ensure the components are at an optimum. Every six months, it is necessary that the chemistry supplier perform a complete analytical breakdown of the proprietary salts and provide the customer with proper adjustments, if necessary. While the proprietary salts are consumed through dragout, the proprietary additives may start to drift over time. This is often dependent upon the technology behind the proprietary blend offered by the chemistry supplier. Modern formulations of proprietary salts experience much less drifting and require much less adjustment by the customer, making the process more user friendly.
To recap, when experiencing dark streaks or deposits out of trivalent chromium plating solutions, common causes to look for are high metallic impurities, complexer imbalance, insufficient or older technology anti-mist additive, and improper balance in proprietary salts. These issues can primarily be avoided through proper setup of the system, regular preventative maintenance and testing, and a solid working relationship between the client and chemistry supplier.
Matt Schario is director of technical services at Columbia Chemical. Visit columbiachemical.com.
Related Content
How to Choose Between Sulfate and Chloride-Based Trivalent Chromium
There are several factors to consider when choosing between sulfate and chloride-based baths for trivalent chromium plating. Mark Schario of Columbia Chemical discusses the differences and what platers should keep in mind when evaluating options.
Read MoreAn Overview of Electroless Nickel Plating
By definition, electroless plating is metal deposition by a controlled chemical reaction.
Read MoreTrivalent Chrome Overview
As the finishing industry begins to move away from the use of hexavalent chromium to trivalent chromium, what factors should finishers consider as they make new investments? Mark Schario, chief technology officer for Columbia Chemical offers a helpful overview of this complicated topic.
Read MoreA Chromium Plating Overview
An overview of decorative and hard chromium electroplating processes.
Read MoreRead Next
Education Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read More
















.jpg;maxWidth=300;quality=90)








