RoHS and ELV Compliant Electroless Nickel
Over the past few years, a number of new environmental directives have come out of Europe and Asia encompassing mainly the automotive and electronics industries.
Over the past few years, a number of new environmental directives have come out of Europe and Asia encompassing mainly the automotive and electronics industries. The purpose of these directives is to reduce the amount of toxic materials entering the environment by limiting the amount of certain toxic substances allowed in a manufactured product and providing for the recyclability of the manufactured product.
| Table 1. Typical Heavy Metal Limits | ||
| Directive |
Limits | |
| ELV1 | Directive 2000/52/EC |
Cadmium <100 ppm Lead <1,000 ppm |
| RoHS2 |
Directive 2002/95/EC |
Cadmium < 100 ppm Lead <1,000 ppm |
| Other OEMs |
<5 ppm Lead <100 ppm |
|
The two major directives affecting industry are the End of Life Vehicle (ELV) Directive and the Restriction of Hazardous Substance (RoHS) Directive. The focus of the ELV Directive is to reduce the amount of heavy metals contained in an automobile and provide for the recyclability of automobile components. The focus of the RoHS Directive is the restriction of the use of hazardous substances in electrical and electronic equipment. Any company interested in selling to the global community must comply with these regulations.
The primary heavy metals addressed in these regulations are cadmium, lead, hexavalent chromium, and mercury. In electroless nickel plating, cadmium and lead are the major concerns since hexavalent chromium and mercury are not typically added to these formulations. The ELV and RoHS Directives specify the limits for cadmium and lead in an electroless nickel deposit at less than 100 and 1,000 ppm, respectively. The ELV Directive initially stated that the cadmium and lead could not be intentionally added even if the final coating met the above limits. It is anticipated that this statement will be dropped from the directive.
Pressure is also coming from certain areas, mainly Japan, requiring no intentionally added cadmium and lead with much lower levels of these heavy metals allowed in the electroless nickel deposit. These lower limits ensure that any detectable amounts of these substances are purely due to impurities in the raw materials used to manufacture the plating chemicals. A summary of the directives and their limits are given below.
Historically, lead and cadmium were added to electroless nickel systems for specific purposes, namely stability and brightness. Electroless nickel baths contain nickel and sodium hypophosphite, a reducing agent, in solution at high operating temperatures. By themselves, uncontrolled reduction of the nickel to nickel powder throughout the solution would occur resulting in a useless system. Addition of lead, in small amounts, stops this reaction, allowing the deposition to occur only on the active substrate immersed into the solution. Lead is a powerful stabilizer, effective at low concentrations, easy to control, and inexpensive. Cadmium is a very good brightener. Like lead, it is very effective at low concentrations, easy to control, and inexpensive. These properties ensured lead and cadmium’s widespread use as basic building blocks in electroless nickel formulations.
The advent of the new environmental directives and OEM requirements has brought new challenges to electroless nickel formulators. The primary challenges are identifying alternative stabilizers and brighteners to the conventionally accepted and proven lead and cadmium. As new formulations are introduced into the field and the technology evolves, metal finishers are experiencing a disparity in supplier and bath performances. Currently, a number of systems do exist which are production proven, and approach and even exceed the stability of conventional leaded systems. In terms of brightness, the bright lead- and cadmium-free systems are approaching the brightness of standard cadmium brightened systems.
The deposit properties of the lead and cadmium free mid and high phosphorus systems have proven to be comparable to the conventional systems (aside from the heavy metal content) and will be illustrated in detail.
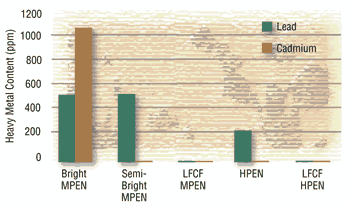
Heavy Metal Content:
In general, bright mid-phosphorus deposits contain approximately 1,200 ppm of cadmium and 550 ppm of lead. Semi-bright mid-phosphorus deposits contain 0 ppm of cadmium and 550 ppm of lead while high-phosphorus deposits contain 0 ppm of cadmium and 250 ppm of lead. The new ELV and RoHS compliant deposits entering the market contain essentially no cadmium or lead. Conventional semi-bright mid-phosphorus and high phosphorus deposits actually comply with the ELV and RoHS Directives, though lead is intentionally added, and are being used to some extent as a “loophole” for the time being until the regulations become stricter or OEMs dictate true lead- and cadmium-free compliance. As stated earlier, many OEMs are now specifying that electroless nickel deposits contain no intentionally added cadmium or lead. This will eliminate the conventional semi-bright mid-phosphorus and high-phosphorus systems from use by platers who supply parts to OEM’s taking the more environmentally friendly route.
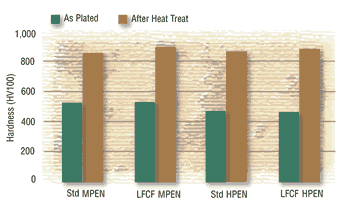
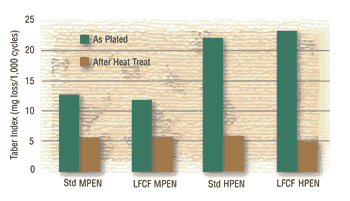
Hardness and Wear:
The hardness values of the new and conventional deposits are essentially identical and fall within the ranges expected for the particular deposit phosphorus contents. As such, the wear indices are also similar.
Corrosion Resistance:
The neutral salt spray resistance between conventional and lead- and cadmium-free deposits is also identical. The NSS resistance depends upon the phosphorus content and thickness of the deposit rather than whether a deposit contains lead or cadmium. This also follows in the nitric acid test for high-phosphorus electroless nickel where deposits from both types of systems pass with no discoloration.
One advantage noted with the new lead- and cadmium-free systems that can translate into better corrosion protection is the much lower tendency of the new stabilizers to cause skip plating. Lead and cadmium, being very powerful compounds, can and do cause edge pullback and skip plating that can result in corrosion failure.
Phosphorus Contents:
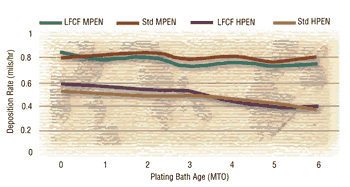
Many of the new lead- and cadmium-free systems have the same basic formulation backbones as the conventional systems, the only differences being the types of stabilizers and additives. As such, the phosphorus contents are comparable and behave similarly over a bath’s life.
Appearance:
In general, the appearance of the deposits obtained from the semi-bright lead and cadmium-free mid-phosphorus and high-phosphorus systems are indistinguishable from the conventional systems. The deposits will be even and semi-bright and will be consistent throughout a bath’s life.
The bright lead- and cadmium-free mid-phosphorus systems vary greatly from supplier to supplier with deposits ranging from semi-bright to almost approaching the level of cadmium brightened systems. There are systems today that are very close in appearance to cadmium brightened systems and maintain their brightness throughout and actually become slightly brighter with bath age. This is unlike cadmium brightened systems which tend to drop off in brightness after approximately 4 MTO’s.
| Table 2. Corrosion Resistance of Deposits | |||
| Deposit |
Mil-C-26074E (>100 hrs NSS) |
AMS 2404D (>48 hrs NSS) |
Nitric Acid (Conc HNO3 30 sec) |
| Std MPEN |
Conforms |
Conforms |
|
| LFCF MPEN |
Conforms |
Conforms |
|
| Std HPEN |
Conforms* |
Conforms* |
Passes |
| LFCF HPEN |
Conforms* |
Conforms* |
Passes |
| *Able to pass 1,000 hrs NSS | |||
Though the above discussion has shown that the performance of the deposits obtained from the new lead and cadmium free systems is comparable to conventional systems, an important factor to the platers is the performance of the electroless nickel plating bath itself. This affects the day-to-day ease of operation and maintenance of the actual electroless nickel solution.
There are systems available currently, which perform very well from a solution standpoint. These systems provide similar make-up components and concentrations, and replenishment components and concentrations. Bath make-up and operation are therefore identical as far as the plater is concerned.
Deposition rates similar to the conventional systems can be obtained from the lead- and cadmium-free systems. Production throughput, therefore, is not affected with the new systems.
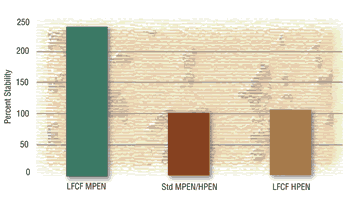
Bath stability is also a primary concern of the plater. An unstable bath affects production throughput, reject rates, and amount of solution maintenance required. Though bath stabilities will vary from supplier to supplier, there are baths with very good stability requiring no more additional maintenance than current systems.
In summary, companies wishing to sell products to the global community must comply with the ELV and RoHS Directives. Electroless nickel platers desiring to meet the strictest of the requirements, either by choice or by OEM requirements, must accomplish this by moving to truly lead- and cadmium-free electroless nickel systems. These systems have been formulated by suppliers in response to the new directives and can provide stable, simple-to-use processes capable of meeting any of the current electroless nickel specifications. The current downside to switching to these processes is that there will be a learning curve by both platers and suppliers as the new technology evolves and improves.
Comparisons of the deposit properties between the conventional electroless nickel systems and the lead- and cadmium-free systems show no distinguishable differences in functional performance. The only advantage noted to date has been the lessened sensitivity of the new systems to bath loading and edge pullback. Conventional and lead- and cadmium-free systems both provide coatings of excellent wear and corrosion resistance for which electroless nickel is well noted. The change to the new lead- and cadmium-free deposits is truly environmentally driven; not functionally driven.
Related Content
Finishing High Reliability, Function Critical Parts
From safety critical automotive and aerospace components to lifesaving medical micro-components and implantable devices, Indiana-based Electro-Spec finishes applications that require zero failure rates.
Read MoreChecon Expands Market Offering with Acquisition of Umicore Electrical Materials USA, Inc.
The company is strategically working to meet future growth opportunities surrounding the use of precious metals and conductive materials like copper and aluminum.
Read MoreRead Next
Episode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read More