SUR/FIN 2023: Capsules from the Technical Sessions I: Emerging Technologies
SUR/FIN 2023 in Cleveland this past June was a resounding success. Due to the efforts of the Technical Activities Committee, ably led by Bill Nebiolo this year, an outstanding program of technical presentations was offered. What follows are summaries of selected presentations from the Emerging Technologies sessions. Additional coverage will be provided in this space in the coming months. The full report can be accessed and printed at short.pfonline.com/NASF23Aug1.
SUR/FIN 2023: Capsules from the Technical Sessions I: Emerging Technologies
Compiled by
Dr. James H. Lindsay, NASF Fellow
NASF Technical Editor
SUR/FIN 2023 in Cleveland this past June was a resounding success. Due to the efforts of the Technical Activities Committee, ably led by Bill Nebiolo this year, an outstanding program of technical presentations was offered. What follows are summaries of selected presentations from the Emerging Technologies sessions. Additional coverage will be provided in this space in the coming months. A printable version of this report can be accessed HERE.
Novel Process of Plating on Polymer Substrates
by
Chris Goode, Chief Technology Officer
Cirrus Materials Science, Auckland, New Zealand
Mr. Goode introduced a novel plating process for a wide range of polymers. The process creates a covalent bond between the metal coating and a wide range of polymer substrates, including but not limited to ABS, PA, PC, PPS, and PEI, as well as engineered polymers reinforced with glass or carbon fibers. The process is inexpensive and sustainable, requiring just a few process steps at low temperature and using benign chemistry.
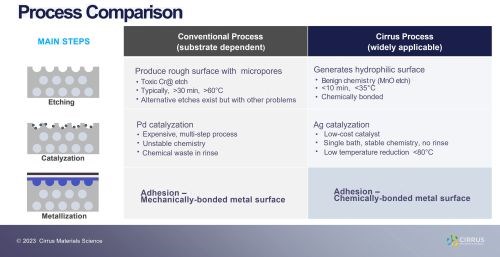
The eco-friendly technology to metallize advanced polymers directly addresses the global need to decarbonize and improve energy efficiency through lightweighting of components in the automotive, electronics and aviation fields. Rapid development of advanced polymer substrates, especially engineered composite polymers, offers lighter, cheaper and easier to manufacture components. However, a metallic coating is often required to provide improved UV or chemical resistance, electrical conductivity and EMI shielding.
Chromic acid etches pose serious toxic hazards; however recently proposed technologies to replace traditional chromic acid etches also have significant drawbacks, given they only work on a limited range of substrates, rely on expensive catalysts, or are challenging to scale up. The novel plating on plastics technology proposed, requires few process steps, operates at low to moderate temperatures, uses benign and cost-effective permanganate chemistry and adopts relatively inexpensive Ag or Cu catalysts. The metallic surfaces produced on ABS exhibit excellent adhesion, classified as 5B following ASTM D3359 with an average peel strength of 19 N/cm following ASTM B533. Moreover, Cirrus successfully applied the technology to metalize hard-to-plate polymers, including PETG, phenolic resins and both glass and carbon fiber-reinforced PPS. Unlike most conventional polymer plating technologies which rely on mechanical interlocking to achieve adequate adhesion, the process develops a chemical bond between the metal layer to the polymer matrix which may simplify composite polymer design or facilitate improved coating of intricate or 3D printed components.
Aspects of High Phosphorus Electroless Nickel Coatings
by
Ambrose Schaffer, Global Product Line Manager-Electroless Nickel,
MacDermid-Enthone, Inc., Canajoharie, New York, USA)
This presentation characterized the unique nature of electroless nickel-phosphorus deposits with alloys over 10.5 wt% P by examining the performance of the deposit with various techniques and tests. While the percentage of phosphorus is used to characterize the deposit, the origin of the unique properties obtained are largely based on its x-ray amorphous nature. The change in properties between crystalline and different levels of amorphous state of the same alloy composition are considered.
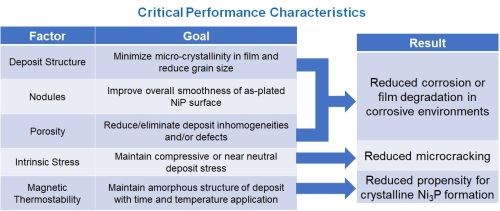
High phosphorus electroless nickel deposits are defined as those which contain more than 10.5 wt% P in the alloy. These deposits are uniquely different than coatings containing less phosphorus and are most commonly utilized for their high level of corrosion resistance and non-magnetic properties. These coatings are especially resistant to oxidizing acids like nitric acid, and as such, deposit quality is often characterized by tests using it. The relatively high concentration of phosphorus in the deposit also leads to other attractive properties such as low intrinsic stress and high ductility. With this, the deposit retains rigidity with a high relative hardness which makes applications such as satellite mirrors and hard disc drives ideal for this deposit property by enabling machining to levels of perfection not met by any other coating or material.
In summary, high phosphorus electroless nickel delivers highly desirable functional properties, including an inert barrier layer, superior corrosion resistance, low stress and good ductility and non-magnetic properties. These higher performance coatings are especially relevant for certain applications, such as hard drives, satellite mirrors, semiconductor tooling and oil and gas components. High phosphorus coatings can be improved with innovative approaches to offer end markets new solutions for ever increasing demands.
Electroless Nickel Pretreatment
by
Alex Beck, Technical Sales
Haviland Products Co., Indianapolis, Indiana, USA
Proper surface preparation is critical for producing continuous and adherent bonds between the part surface and the plated metal. Any debris/oils/ contamination must be removed from the surface so that the aforementioned bonds may form with the least amount of distortion. Some surfaces may require further preparation to allow the electroless nickel deposition reaction to occur. This is particularly important when it comes to deposition onto an aluminum surface. Substrates that can be plated with electroless nickel include steel/steel alloys, aluminum/aluminum alloys, copper/copper alloys, zinc die cast and nonmetallic surfaces. Steel and aluminum are by far the most used substrates. This presentation discussed the unique steps required to properly prepare and activate steel and aluminum substrates for electroless nickel plating.
Steel and aluminum alloys must undergo separate pretreatment regimens. It is important to note that each process step is followed by a thorough water rinse.
Steel preparation
The elements of steel preparation are: (1) alkaline soak cleaning, (2) electrocleaning, (3) acid dip or electro-acid and (4) a Woods nickel strike, which is alloy dependent. Steel alloy parts arrive coated with oils and soils, including dirt from transportation and warehousing, oils to prevent flash corrosion, buffing compounds, forming lubricants and other proprietary rust preventatives.
Alkaline soak cleaners from early in the industry consisted of KOH and animal fats. Solid and liquid alkaline cleaners are commonly used in the industry today to remove oils and soils. Cleaning technology consists of NaOH, KOH, surfactants/wetting agents, solvents/emulsifiers, chelating agents, phosphates, etc. Most cleaners operate at 130°F to 200°F, and use both chemical (saponification, emulsification, wetting agents) and mechanical (heat, agitation, electrolysis (for electrocleaners)) to remove contaminants.
Typically, acid dips consist of hydrochloric acid, to remove remaining alkaline and oxide films that would otherwise disrupt the nickel strike deposition. In the case of an electro-acid, the work is cathodic. Electro-acids provide greater physical cleaning action through increased gassing, and help activate stainless steel parts, with higher nickel contents. More sulfate / sulfamate-based chemistry is used, as chlorine gas, generated with HCl, is not a problem.
The Wood’s nickel strike (nickel chloride, 25 oz/gal; HCl, 11 oz/gal; 60-80°F; 20-30 A/ft2) provides an active layer on substrate ready for electroless nickel. A rinse is essential so as to not contaminate the electroless nickel bath.
Aluminum preparation
Aluminum preparation involves: (1) acid or caustic etch, (2) de-smut or de-oxidation and (3) a zincate or double zincate. Aluminum parts naturally form an oxide layer and usually don’t need any protective oils to maintain surface integrity.
An acidic etch cleans as well as activates the aluminum surface. It removes oils, oxides, drawing lubes, buffing compounds, etc. Phosphoric acid-based, it is blended with dispersants, wetting agents, detergents, etc., and is more suited for subsequent bright dipping or anodizing. A caustic etch, on the other hand, scrubs the surface of the part to produce a uniform surface and remove impurities. It actively dissolves aluminum and creates extremely small pits on the surface.
The de-smut or de-oxidation process removes the oxide/smut film built up by the etching process. It is a nitric-based bath and may contain sulfuric acid and bifluoride. After this stage, the parts should be water break free.
The zincate process prevents the oxide film from reforming on the active aluminum surface, allowing for proper adhesion to the substrate. It usually consists of zinc oxide, various metal oxides, and caustic soda, with iron, nickel and copper additives. It typically operates at 60-85°F for 30-60 seconds. For a double zincate, one can strip in 50% nitric acid and followed by a second dip. The reaction occurs more quickly and evenly through the second cycle. The autocatalytic deposition reaction produces a deposit thickness of 2-20 µm.
Pathways for Sustainability in Electroless Nickel Processes
by
Douglas Hughes, Product Manager, Wear Resistant Coatings, North America
MacDermid-Enthone, Inc., Southington, Connecticut, USA
This presentation discusses sustainability strategies of modern electroless nickel processes. Environment, Social, and Governance (ESG) has become a major focus of businesses operating in today’s ever-changing corporate landscape. For metal finishers, sustainability is a key component to maintaining a high ESG rating. In this presentation, we look at pathways for sustainability in electroless nickel processes, examples of sustainable processes currently available to the market, and the impacts of ESG on electroless nickel innovation strategies.
The drivers for sustainability in electroless nickel (EN) processes are numerous. They include (1) increasing regulatory scrutiny, currently with PFAS concerns, (2) the ESG initiatives themselves, (3) competition for resources, including nickel and (4) climate change. In this presentation, several examples of sustainable EN were discussed.
PFAS-free EN-PTFE
Current technology involves the codeposition of sub-micron particles of polytetrafluoroethylene (PTFE) within an EN deposit, enhancing the lubricity of the deposit. Until recently, fluorinated surfactants were considered essential to enable PTFE build-in and dispersion stability. Traditionally produced PTFE powders typically show traces of PFOA in the range of or above 25 ppb. Fluorinated surfactants, classified as PFAS are becoming heavily restricted, both in Europe and North America. PFOA trace amount contamination is already regulated. One substitute, EN hBN (hexagonal boron nitrate) dispersion coating is a costly and complex alternative to EN PTFE.
REACH, RoHS and other regulatory regimens have motivated the development of regulatory compliant (RC) EN PTFE processes to eliminate fluorinated surfactants in PTFE dispersions. Proprietary processes are available which provide unchanged performance and properties, which overcome the regulatory issues. The processes serve as a drop-in replacement for compliance on PFOA/PFOS/PFAS, are free of fluorinated surfactants and trace PFOA contamination, involve no equipment changes, with economic operation and costs, good plating rates, and meeting existing standards.
Reduced ion low metal EN
The sustainability motivation here is to reduce nickel concentration and other bulk components. In particular, a process was described which operated at 3 g/L Ni versus 6 g/L Ni, a 50% reduction. The result is reduced material in the waste stream and rinses, and reduced nickel exposure and emissions from the bath. Phosphorus contents range from 6 to 9 wt%, and the deposit stress remains compressive through six metal turnovers. All formulations produce bright, uniform deposits and meet >1000 hr of NSS at 25 μm thickness. Operational cost savings of 10% were reported. Ten years of field experience indicate successful results.
Reduced metal content has also seen success with high phosphorus systems (>10.5 wt%). Compressive deposit stresses are produced up to five metal turnovers, and all formulations produced a semibright uniform deposit. Five years of field experience have shown the processes to be successful. Current research may determine if EN solutions can be further diluted.
Ammonia-free EN strike
An electroless nickel strike serves as an intermediate thin EN layer between the zincate and final EN deposit. With a slightly alkaline pH, much less zinc from the zincate layer is dissolved. Accumulation of life limiting zinc in the final EN process is minimized for maximum solution life. Using an EN strike enhances adhesion and uniform appearance of the final EN layer. However, ammonia in the formulation presents problems in workplace quality and safety.
Ammonia is a severe respiratory irritant. It can cause severe skin burns and damage to the eyes. The odor threshold is 5 to 50 ppm. Exposure to ammonia >300 ppm poses an immediate risk to health and safety. Exposure to high concentrations of ammonia in the air causes immediate burning of the nose, throat and respiratory tract.
Here, sustainability development motivation involves the elimination of ammonia from the baths. Such a process improves wastewater treatment effectiveness and facilitates <0.1 mg/L nickel during effluent treatment to meet increasingly tighter global wastewater regulations. It is lead and cadmium free to meet another critical sustainability objective, while maintaining the performance properties of established ammonia-based electroless nickel strike processes.
An ammonia-free process was described which exhibits a deposition rate comparable to that of a conventional strike. Reduced stress in the strike layer was found to improve adhesion, while a higher phosphorus content slightly increased the passivation rate of the strike layer. The result was a stable process with greater pH stability due to the lack of volatile ammonia. Two years of field experience has shown positive results.
In summary, viable, sustainable EN technologies are currently available in the marketplace. The increasingly restrictive regulatory landscape will accelerate demand for sustainable EN technologies. Future EN innovation efforts will be strongly influenced by an evolving sustainability landscape.
Encapsulated Plating: Expanding Selective Plating
Capabilities for Non-Line of Sight Areas
by
Danijela Milosevic, R&D Manager
Sifco, ASC, Independence, Ohio, USA
Environmental degradation of metal surfaces can be caused by fatigue, wear, creep and corrosion. The electroplating process can be used to offer improved corrosion resistance, durability, reduced friction and improved conductivity. Ultimately, plating can be used to repair, enhance and increase the life cycle of a product, thereby improving performance and reducing component failure.
As technology changes, selective plating applications are becoming more challenging. The need to apply localized deposits and coatings onto complex geometries or areas that are deeply recessed in components has required a shift from traditional brush and tank plating to that of encapsulated plating technology.
Selective flow, or encapsulated plating, is a closed loop electroplating process in which the anode and workpiece are completely encapsulated only along the areas of interest, thus eliminating the need for large tanks, laborious masking, and operator exposure to potentially hazardous effluents. Key design elements include sealing at the interface of plating tool and workpiece, anode-to-cathode gap, solution flow rates and exchanges, anode materials and electrolyte plating parameters. An optimized design and controlled plating process provide for a higher quality deposit that is repeatable and reproducible from part to part.
This presentation discusses the challenges of complex components and deep recesses, and demonstrates how selective flow plating has been successfully used in critical aerospace and oil and gas applications to apply deposits for corrosion protection and anti-galling.
The critical parameter for brush plating is anode-to-cathode movement. When neither the anode nor the workpiece can be rotated/moved, encapsulated flow plating provides an alternative, where the solution speed provides the brushing action. Potential applications include axi-symmetric geometries such as ID/OD’s, channels, and annuli. They can be non-light-of-sight, have non-axisymmetric access, or complex geometries. The surface, however, must be suitable for sealing with gaskets.

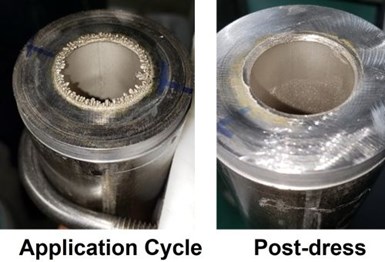
A heat exchanger application was studied involving the plating of internal bores with 5 mils of nickel. The design concept involved the use of a single anode, consisting of a mixed metal oxide on titanium, for all surface prep and plating steps. Robust equipment design was required to handle a variety of applications. The concept is shown in the figure below. A single bore mock-up is shown schematically (center) and under test (right). The steel pipe was 12” long with an ID of 1”.
The objective was to evaluate the deposit characteristics under a variety of plating conditions by varying solution flow rate, current density and anode-to-cathode gap with a target thickness of 0.040 inches. A factorial study was undertaken, where solution flow rates were 0.5 m/sec (low flow) and 2.0 m/sec (high flow). Plating current densities were 1.5-2.0 A/in2 (low CD) and 4.0 A/in2 (high CD). The anode-to-cathode gap levels were 1/8 and ¼ inch.
In general, during plating, there was a steady decline in voltage after approximately 5 min before an abrupt drop to ~1 V, at which point the application was stopped. Examination of the ends of the pipe showed a nodular build-up which negatively affected the current-voltage distribution. The edges were dressed and deburred, and plating was resumed. If required, this process was repeated for a second or third application to acquire the 0.040-inch target thickness. This procedure was used as each of the factorial study specimens was prepared.
The results showed the high flow and low current condition yielded smooth deposits. Deposit thickness was >40 mils and was possible without additional machining in between. Slightly harder deposits were observed. The low flow and high current conditions yielded rough and pitted deposits. Slightly softer deposits were observed. High builds were not recommended unless there was an additional machining application in between the application cycles. Plating duration was limited by the anode-to-cathode gap. The larger gap (1/4 inch) increased plating duration by 50% compared to the 1/8-inch gap. The 40 mil target was achieved in two applications at the ¼-inch gap compared to three applications at the 1/8-inch gap.
Certain advantages were realized with the encapsulated plating method. There was minimal masking, and the process was less labor-intensive as compared to traditional brush plating. There was no need to immerse entire parts in corrosive solutions, and minimal waste generation was noted. It was a clean process, a closed-loop system, with minimal exposure to fumes. Controlled plating applications were repeatable and reproducible, and fast plating rates were possible.
Related Content
Attention to Measurement
English vs. Metric — which should you use? Ron Kinne of Haviland Enterprises Inc. explains why you need a firm understanding of both for measuring various aspects of plating processes.
Read MoreBYK-Gardner Offers Protective Coatings Field Test Kit
New field kit includes tools for a proper assessment of environmental conditions prior to coating applications and film thickness evaluation.
Read MorePowder Coating Overcomes Post Forming
Six Sigma methodology, open communication, and collaboration produce results for leading boat manufacturer.
Read MoreBusiness Card Doubles as a Wet Film Thickness Gauge
The customizable card can be used to measure wet film thickness.
Read MoreRead Next
A ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read More













.jpg;maxWidth=300;quality=90)







