The Study of Copper Anodes in Acid and Cyanide Plating Baths
The 1956 Carl E. Huessner Gold Medal Award was given to Charles Faust and William H Safranek for Best Paper appearing in Plating or the AES Technical Proceedings in 1955, and their paper is republished here in a series on the AES/AESF/NASF Best Paper Awards. Their work involves an evaluation of anodes for copper plating at the time when OFHC anodes were first emerging in use.
by
Charles L. Faust & William H. Safranek
Battelle Memorial Institute
Columbus, Ohio, USA.
The 1956 Carl E. Huessner Gold Medal Award for Best Paper appearing in Plating or the AES Technical Proceedings in 1955
Originally published as C.L. Faust and W.H. Safranek, AES 42nd Annual Technical Proceedings, 42, 193-198 (1955)
Editor's Note: This paper is part of a series on the AES/AESF/NASF Best Paper Awards. In 1955, Charles L. Faust and William H. Safranek received the Carl E. Huessner Gold Medal Award for Best Paper appearing in Plating or the AES Technical Proceedings. A printable PDF version is available by clicking HERE.
Introduction
For several years after high-efficiency copper cyanide baths were introduced, electrolytic copper was preferred for anodes. The electrolytic copper then was the only high-purity metal supplied by reliable sources.
High-purity cast anodes (OFHC) became available about two years ago [~1953], overcoming the disadvantage of the electrolytic copper with respect to fastening hooks. The cast anodes dissolve uniformly, thus, creating less scrap than electrolytic copper. Furthermore, the high-purity cast anodes are supplied in lengths up to 90 inches, whereas electrolytic copper slabs are only 36 inches long. Most anodes used for plating need to be longer than 36 inches.
More than two million pounds of the high-purity castings have been used for copper plating since they were introduced. Several users have reported better results with the new cast form, by comparison with electrolytic copper. Particle roughness has been reduced. As a result, diaphragms have been eliminated in some installations.
The high-purity cast anodes were compared with electrolytic copper and with other types of anodes in laboratory and pilot-plant baths to provide information needed for commercial operation. This report deals with the laboratory and pilot-plant data.
Density and purity of anodes
The density, purity, and grain size of the anodes included in this study are listed in Table 1. The impurity contents given in Table 2 were the basis for calculating the purity, in accordance with the customary procedure for such an estimation.
The oxygen-free anodes and the rolled bars were denser than the electrolytic copper or the conventional castings. The porosity in the electrolytic and the conventional cast copper is associated with irregular dissolution in copper-plating baths, leading to the formation of small anode particles that contribute to "shelf" roughness in copper electroplates.
Table 1 - Density, purity and grain size of copper anodes.
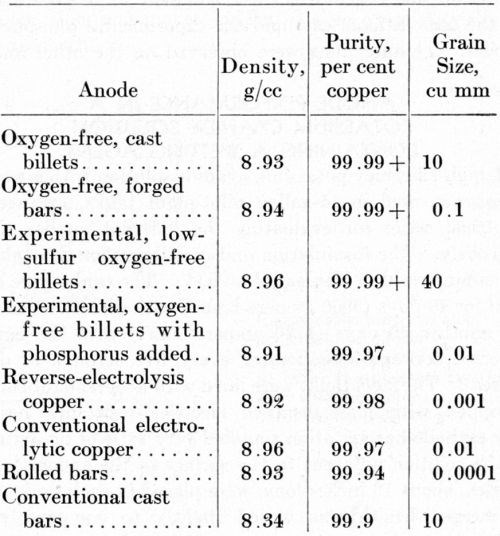
Table 2 - Impurity contents of copper anodes.
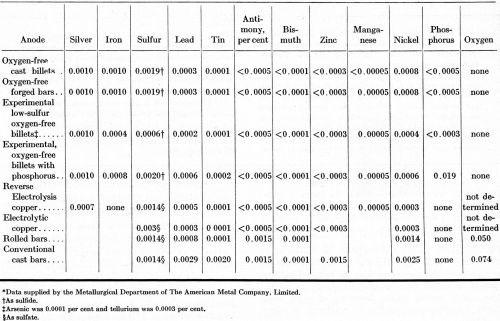
The oxygen-free anodes were the purest among the eight forms of copper investigated. The metallic impurity contents of the oxygen-free cast and forged anodes were as low as the metallic impurities in either of the two electrolytic grades. The experimental oxygen-free anodes with a low-sulfur content contained less metallic impurities than the electrolytic copper.
A set of OFHC brand copper anodes containing 0.019% phosphorus was included in this study in view of the favorable results reported a year ago on the effect of adding phosphorus to cast copper anodes used in copper-sulfate baths [R.P. Nevers, R.L. Hungerford and E.W. Palmer, “Effect of Anode Composition in Acid Plating Baths," Technical Proceedings, American Electroplaters' Society, 41, (1954)]. Except for the deliberate phosphorus addition (added as phosphor copper), the purity of this set of anodes was equal to the purity of the commercial, oxygen-free anodes.
The reverse-electrolysis copper is produced by reversing the current for a short time after the starting cathodes are immersed in the electrorefining cells in order to improve the adherence of the subsequent cathode deposit on the starting sheets. As a result of this procedure, reverse-electrolysis copper does not separate into two layers, as ordinary electrolytic copper does during use in copper electroplating baths.
Grain size was determined by measuring the dimensions of grains in representative longitudinal and transverse sections, after polishing and etching in accordance with customary metallographic techniques. The results of this study confirm previous indications that grain size, per se, does not influence the behavior of copper anodes.
Anode performance in high-efficiency potassium cyanide solution with no addition agents
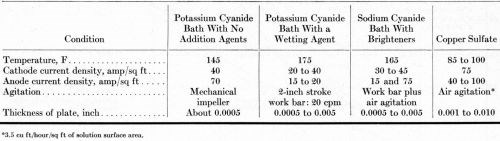
Each of the anodes listed in Table 1 was evaluated in a preliminary test in a high-efficiency potassium cyanide bath containing no addition agents, except Rochelle salts. The formulation of this solution and the operating conditions are summarized in Tables 3 and 4. The cathodes were flat panels with the bottom half-inch bent out on a horizontal plane, forming a shelf. No diaphragms separated the anodes from the cathodes. Plating solution was filtered into the plating tanks but was not filtered subsequently. The mechanical impellers supplied agitation at both the cathodes and the anodes.
With the oxygen-free cast and forged anodes, copper plates, averaging about 0.0005 inch thick, contained very few or no nodules. Furthermore, the solutions remained clear and free of anode particles. Deposits produced with the reverse-electrolysis copper and the experimental phosphorized anodes consistently contained a few nodules. The conventional electrolytic copper and the experimental low-sulfur anodes contributed several nodules to each deposit. Very rough nodular deposits were obtained with the rolled and conventional cast bars.
Each anode was covered with a brown or black film within a few minutes after the current was started. When the operation was concluded, thick adherent films were evident on the conventional cast and the experimental phosphorized anodes. Thinner films were observed on the other anodes.
Anode performance in a potassium cyanide solution containing a wetting agent
A high-efficiency potassium cyanide solution with a wetting agent was used in 15-gallon pilot-plant tanks, arranged in electrical series for evaluating the different anodes simultaneously. The formulation and conditions for this solution are summarized in Tables 3 and 4. The tanks were operated for 20 days (3600 A-hr) with direct current and for another 20 days (4120 A-hr) with the current reversed for one second after every five seconds of direct current. The steel tanks were lined with neoprene rubber and equipped with glass-sheathed electrical-resistance heaters. The cathode-bar agitation supplied very little or no agitation of the solution adjacent to the surface of the anodes. Four anodes, about 13 inches long, were placed in each tank. The submerged length was varied slightly to compensate for differences in circumference, equalizing the anode current density in each tank.
Table 3 - Bath formulations of copper cyanide baths.
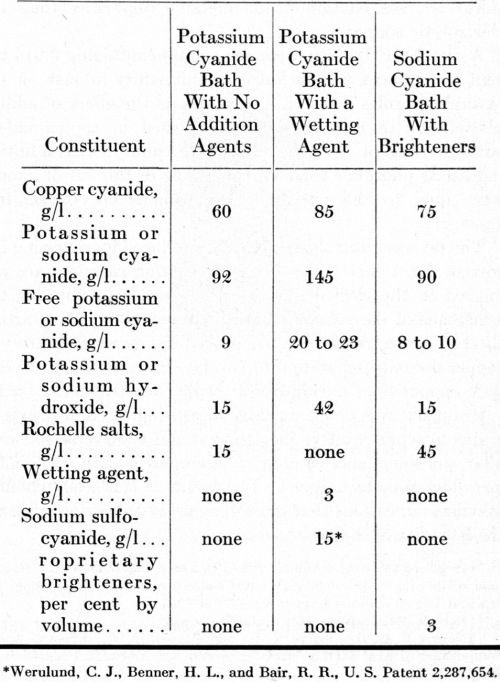
Table 4 - Operating conditions for copper plating baths.
Voltage data at increasing anode current densities with direct and PR current are given in Figs. 1 and 2. A slightly lower voltage was observed for the electrolytic copper anodes relative to other anodes. However, the depolarizing influence of the periodic current reversal has more significance. Anodes that began to polarize at about 20 A/ft2 with direct current showed no tendency to polarize with PR current until the current density exceeded 30 A/ft2.

Figure 1 - Relationship between tank potential and anode current density at approximately 170 and 176°F in a high-speed potassium cyanide bath with direct current (3.0 oz.gal free potassium cyanide).
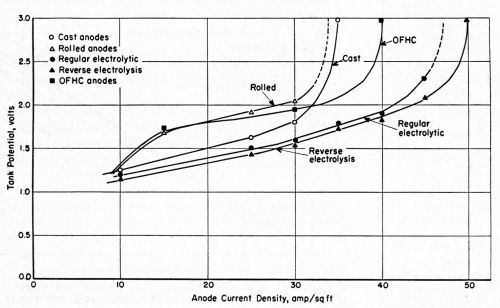
Figure 2 - Relationship between tank potential and anode current density at approximately 175 and 180°F in a potassium cyanide bath with the current periodically reversed.
After the voltage measurements, the baths were operated steadily with an anode current density of 15 to 20 A/ft2, simulating commercial conditions. Potassium cyanide additions were made once or twice a week to adjust the free cyanide concentration to 23 g/L. Supplementary wetting agent (2 g/L) was added to each bath after a few days of operation to reduce the surface tension to 35 dynes/cm. The solution was not filtered after it was initially prepared and filtered into each plating tank. No diaphragms were used.
Electroplates, about 0.0005 inch thick, were deposited at least once a day on buffed die castings cleaned and plated in a low-efficiency "strike" bath according to the customary procedure. At other times, steel panels were plated after cleaning and striking. The thickness of such plate usually was about 0.001 inch, but thicker plates were deposited at regular intervals. The plates on the die castings were consistently bright and smooth. A very few nodules generally were observed in the thicker deposits on the steel. However, all the plates were about equal in smoothness with the agitation conditions used in this study. No significant difference in ductility was detected by bend tests. PR current showed no tendency to improve the smoothness of the thick deposits.
Anode efficiency and sludging data are given in Table 5. With PR current, the anode current efficiency for all anodes was more than 100 per cent. With direct current, only the electrolytic copper dissolved with an anode efficiency exceeding 100 per cent.
Table 5 - Anode efficiency and sludging data on operating potassium cyanide baths with OFHC, cast, rolled, regular-electrolytic and reverse-electrolysis anodes.
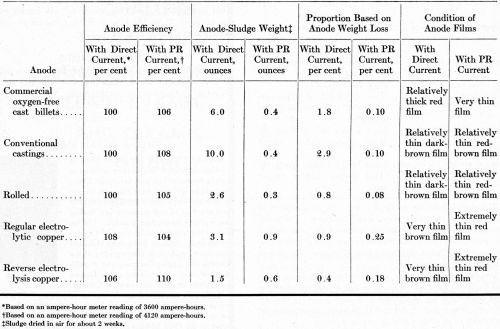
Operation with direct current caused more anode sludge than operation with PR current. Thus, PR must assist the dissolution of copper oxide that tends to sludge out on the anode as a result of depletion of free cyanide in the anode film. Any undissolved copper particles that became detached from the anodes during operation evidently were carried down with the sludge, because only very few nodules were evident on the cathode deposits. If the solutions had been agitated more, roughness might have nucleated either from undissolved copper particles or undissolved oxide particles.
Copper, iron, lead, and magnesium were identified in the sludge from each tank. Other trace impurities, including silver, zinc and nickel, evidently were completely dissolved in the bath.
Anode performance in air-agitated sodium cyanide baths
Sodium cyanide baths with the formulation given in Table 3 were agitated with air, chiefly to supply the turbulence necessary for producing bright plate with the proprietary brighteners at hand. The anode current density was increased from 20 to 80 A/ft2 with no evidence of anode polarization. The voltage data are shown in Fig. 3. Only the phosphorized anodes acquired an anode film. The other anodes remained clean at all times.
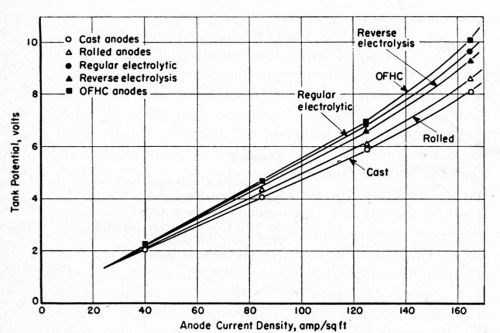
Figure 3 - Relationship between tank potential on anode current density at 156 to 158°F in air-agitated sodium cyanide baths with a free cyanide concentration of 10 g/L (165°F).
Steady operation of 15-gallon baths for 22 days (2400 A-hr) with an anode current density of 15 to 20 A/ft2 caused roughening of the surface of all anodes. Very small copper particles could be detached from many of the anodes by rubbing their surface with a knife edge. Such particles evidently caused the nodules that were observed on many of the cathode electrodeposits. The electroplates were 0.0005 inch thick on zinc die castings and 0.001 inch thick on steel panels.
The smoothest plates were produced in the bath operated with the oxygen-free cast anodes. Very few or no nodules appeared on most of these plates. By comparison, many large and small nodules were evident on all of the plates obtained with the other anodes.
Only about 1 gram of sludge was filtered from each bath after dissolving more than 12 pounds of copper from the anodes by electrolytic operation. This sludge contained chiefly aluminum and calcium oxides, evidently introduced with the water used for bath volume makeup. This sludge evidently does not cause roughness, because smooth plate was produced in the bath equipped with the oxygen-free castings, despite the sludge suspended in the solution by the air agitation.
The nodules observed on the deposits obtained in the other baths are believed to have been caused by undissolved copper particles that became detached from the anodes. Preferential grain-boundary corrosion might be the cause for the uneven dissolution of the surface of the copper anodes.
With steady operation at an anode current density of 75 A/ft2, the smoothness of plates produced with any individual set of anodes was not consistent. However, fewer and smaller nodules usually were observed on deposits produced with the oxygen-free anodes, compared with the other electroplates. The forged oxygen-free anodes appeared to be the best anodes, with respect to particle roughness. The experimental low-sulfur, and commercial oxygen-free, cast anodes were nearly as good as the forged anodes. Electrolytic, rolled, conventional cast, and phosphorized anodes caused nodular deposits during a two-week operating period.
The results of operating the air-agitated sodium cyanide baths with both a high and a low anode current density are summarized in Table 6. In general, the plates obtained with an anode current density of 75 A/ft2 were smoother than the deposits produced with an anode current density of 15 A/ft2. The anodes dissolved very smoothly at 75 A/ft2. Their surfaces were bright as well as smooth. Thus, anode dissolution was improved by increasing the current density in the air-agitated solution. This behavior may be the key to the development of very high-speed plating at a higher rate than that used commercially today.
Table 6 - Performance of copper anodes in air-agitated, high-efficiency sodium cyanide baths.
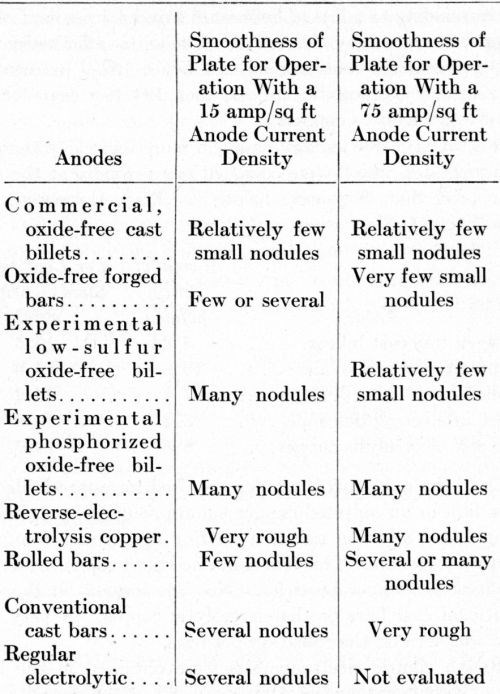
The anode efficiency for all anodes at 75 A/ft2 was about 110%; at 15 A/ft2, it was 103 to 104%. The consumption of sodium cyanide averaged 0.7 lb./l,000 A-hr, equivalent to about 0.25 lb. of sodium cyanide/lb. of copper dissolved from the anodes.
After operation with an anode current density of 15 A/ft2, and before increasing the anode current density to 75 A/ft2, the copper plating solution was contaminated accidentally with a very small amount of silver cyanide plating solution. The silver caused very rough deposits, until it was removed by dummy plating for about three hours. The results indicate that silver is a harmful impurity.
Anode performance in copper sulfate plating baths
Five 15-gallon air-agitated copper sulfate baths were operated for 26 days (19,340 A-hr) to compare the oxygen-free cast anodes with rolled, reverse-electrolysis, conventional electrolytic, and conventional cast copper anodes. The solution was prepared with 188 g/L copper sulfate (25 oz/gal CuSO4•5H2O), 75 g/L sulfuric acid (10 oz/gal H2SO4) and 1.0 g/L phenol sulfonic acid. The solution was filtered into each tank but was not filtered thereafter. The temperature usually was maintained at 90 ± 5°F.
The relationship between the tank voltage and the anode current density is shown in Fig. 4. With turbulent agitation of the solution adjacent to the anode surfaces, the anodes did not polarize appreciably when the current density was as high as 160 A/ft2.
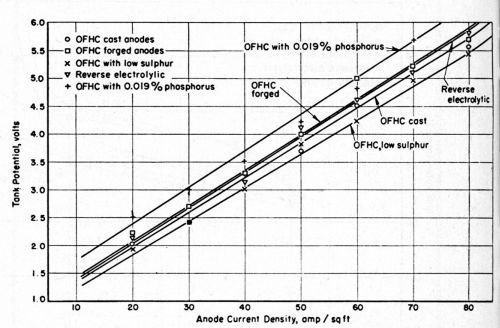
Figure 4 - Relationship between tank potential and anode current density at 100°F in a copper sulfate-sulfuric acid electrolyte.
Most of the time, six 13-inch long anodes were installed in each tank, giving an anode current density of 40 A/ft2 before the surface area was decreased by dissolution. Table 4 details the operating conditions. No anode bags or diaphragms were used.
The appearance of the anode films was influenced by the anode impurities. The films appeared as follows:
| Anode | Anode Film |
| Oxygen-free cast billets | Light brown film of finely divided copper and/or copper oxide |
| Conventional cast ovals | Intermediate brown film of copper particles and/or copper oxide and impurities |
| Rolled bars | Heavy black film of copper particles and/or copper oxide and impurities |
| Regular electrolytic copper | Light brown film of finely divided copper and/or copper oxide |
| Reverse electrolysis copper | Light brown film of finely divided copper and/or copper oxide |
The sludge from the anode film particles accumulated at the bottom of the tanks where it did no harm. The rolled and the conventional cast anodes produced the most sludge; the oxygen-free billets and the electrolytic anodes introduced less sludge. Sludge represents loss of copper that is not usable by the electroplater.
Despite the differences in the character of the anode films and the amounts of accumulated sludge, no major differences were observed in the smoothness or appearance of the cathode deposits. Plate about 0.001 inch thick was relatively smooth; 0.010-inch thick plate was consistently nodular.
The oxygen-free billets and the rolled bars dissolved with an efficiency of 101%, so the copper concentration of solutions equipped with these anodes increased only very slightly. By comparison, the copper sulfate content of the other baths increased by 5 oz/gal or more during the time that about 50 lb. of copper was dissolved from the anodes, corresponding to a loss of between 2.6 and 4.2% of the copper consumed by dissolution. The anode efficiencies were 103% for conventional cast ovals, 102+ % for the regular electrolytic anodes, and 104 % for the reverse-electrolysis copper.
The oxygen-free anodes dissolved more uniformly than the other anodes, which were corroded more rapidly at the solution level than they were below it. Thus, the oxygen-free anodes created less scrap, as follows:
| Anode | Weight of Scrap (lb.) | % Scrap, based on original weight |
| Oxygen-free cast billets | 5.25 | 10.5 |
| Conventional cast ovals | 10.0 | 13.9 |
| Rolled bars | 7.7 | 13.2 |
| Regular electrolytic copper | 8.4 | 14.9 |
| Reverse-electrolysis copper | 8.3 | 14.7 |
Only the oxygen-free anodes acquired an appreciable surface film in air-agitated copper sulfate solution containing a proprietary addition agent, a wetting agent, and 4 mg/L of hydrochloric acid. This film contained both copper oxide and undissolved copper particles. No film formed on the conventional cast bars or the electrolytic copper. A very thin film was seen on the rolled copper bars.
Rough copper electrodeposits were obtained in the bath with the conventional cast anodes. All of the deposits produced with the rolled anodes and the electrolytic copper anodes were nodular consistently. No nodules occurred by operating with the commercial-brand oxygen-free copper anodes. The thickness of each copper plate was between 0.001 and 0.0013 inch. The cathode current density was in the range of 75 to 100 A/ft2, and the anode current density was about 35 A/ft2.
Acknowledgments
The authors acknowledge with appreciation the permission of The American Metal Company, Limited, and Battelle Memorial Institute to publish this paper.
About the authors
NOTE: The biographical sketches below were those composed at the time of publication. In later years, the authors gained additional renown, both becoming recipients of the AES/AESF Scientific Achievement Award (Dr. Faust in 1960; Mr. Safranek in 1979), among other accomplishments.


Related Content
Liquid Chrome Vs. Chromic Acid Flake
Contemplating how to continue offering chromic acid services in an increasingly stringent regulatory world? Liquid chrome products may be the solution you’re looking for.
Read MoreSuccessful South African Plater Beating the Odds
Remaining focused on quality and reliability, Team Plating Works stays profitable in a volatile and challenging economy.
Read MoreAn Overview of Electroless Nickel Plating
By definition, electroless plating is metal deposition by a controlled chemical reaction.
Read MoreProducts Finishing Reveals 2023 Qualifying Top Shops
Each year PF conducts its Top Shops Benchmarking Survey, offering shops a tool to better understand their overall performance in the industry. The program also recognizes shops that meet a set of criteria to qualify as Top Shops.
Read MoreRead Next
Episode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read More

















.jpg;maxWidth=300;quality=90)







