Toward Automatic Control of Nickel Plating Processes
Technological advances in electronics manufacturing and the design of automobiles have brought about a trend toward very narrow specifications and increasingly stringent requirements on the quality of electrodeposited layers of nickel.
Editor's Note: Originally published as J. W. Böcker, et al., Plating and Surface Finishing, 79 (3), 63-69 (1992), this paper received the AESF Gold Medal Award for Best Paper published in Plating & Surface Finishing for the year 1992. A printable version of this paper can be accessed HERE.
ABSTRACT: Technological advances in electronics manufacturing and the design of automobiles, to name just two of the most striking examples, have brought about a trend toward very narrow specifications and increasingly stringent requirements on the quality of electrodeposited layers of nickel. The most frequently used formulations of nickel plating solutions (Watts nickel, nickel and nickel-cobalt sulfamate) have been the subject of considerable research and development activity in the last three decades.1-5
The key parameters of nickel plating are now relatively well understood. With one notable exception, all are measurable by simple techniques. Correspondingly, numerous methods of continuous monitoring have been demonstrated and described in the literature.5-10 Reliable deposition of high-quality layers from nickel plating solutions, however, requires continuous and simultaneous control of key parameters. Concentration of bath additives and their decomposition products during plating are, therefore, of particular interest.
Internal stress-reducing reagents that contain sulfur atoms may be divided into two groups, according to their chemical behavior in the nickel plating solutions. First, there are compounds capable of providing traces of sulfur for electrodeposited nickel without causing detectable accumulation of the corresponding decomposition products. Examples are 1,3,6-naphthalenetrisulfonic acid (NTSA) and p-toluene sulfonamide (TSA). Sulfur deposition by NTSA and TSA generates, respectively, naphthalene and toluene as byproducts of the cathodic reaction.11 Both compounds are relatively volatile and easily removed by evaporation from nickel baths, which are usually maintained at elevated temperature. Photometric evaluation of the UV absorption at 254 nm provides both a selective and a sensitive method for monitoring the mg/L-levels of NTSA and TSA. As a consequence, the decomposition-product-free nickel baths may be considered amenable to process monitoring by means of low-cost and easy-to-apply monitoring techniques.
An important representative of the second group of stress-reducing, sulfur-containing compounds is saccharin. The electrolytic cathode reaction has been recognized as having several alternate paths (Fig. 1). Each of these reaction paths results in detectable levels of organic compounds delivered to the plating solution. According to a comprehensive study carried out in our laboratory, saccharin is to be considered one of the most effective and universal stress-reducing agents. Not only is it capable of influencing internal stress at levels much lower than NTSA and TSA, but it exceeds these compounds in versatility as well. At higher current densities and in certain bath formulas (e.g., Ni-Co), saccharin is the only known internal stress-reducing agent available. Simultaneous monitoring of saccharin and its decomposition products is advantageously carried out by High-Performance Liquid Chromatography (HPLC). During an investigation to be described later, the above method was optimized for use under process conditions, and correlations of liquid chromatography results with important mechanical properties of electrodeposited nickel layers were obtained.
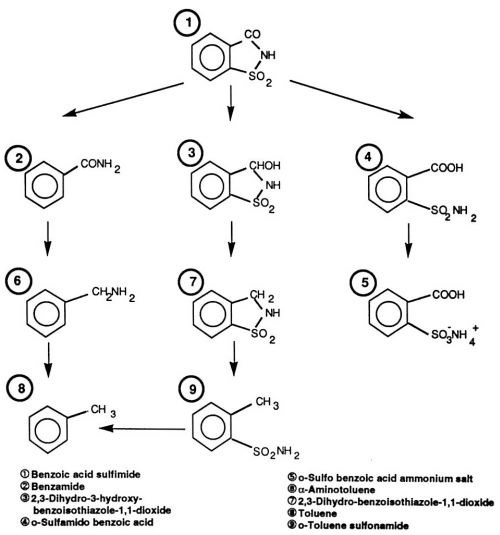
Figure 1 - Reaction paths for the decomposition of saccharin (benzoic acid sulfimide) in nickel electrolyte solutions.
Experimental procedure
All electrodeposition experiments were carried out in a glass container (19 × 14 × 13 cm). The bath temperature was regulated to ±0.5°C and gentle agitation was provided by means of a magnetic stirrer. The volume of the plating solutions was kept constant at 3.0 liters by automatic feedback circuitry connecting a level indicator with a small pump capable of adding measured volumes of deionized water to compensate for losses by evaporation. Flat substrate cathodes were made of electropolished stainless steel and placed at a distance of 17 cm from the nickel anode. Except for the area of exactly 10 × 10 cm, the cathode surface was masked by a chemically resistant insulating tape. Prior to the experiments, the cathodes were cleaned with Vienna Chalk to remove traces of oils introduced by finger contact and from other sources.
The initial series of experiments (internal stress vs. concentration of stress reducers; mechanical properties of nickel vs. pH and vs. current density, etc.) was carried out with a freshly made-up plating solution for each of the data points. In the second set of experiments, the concentration of inorganic bath constituents was held constant, while the levels of organics were allowed to fluctuate as a consequence of a plating process documented in terms of A-hr/L. The size of the samples taken from the baths was one mL or less, which kept the concentration changes as a result of the sampling below the precision value of the evaluation procedures.
In still another evaluation, the inorganic constituents were again kept constant and the effect of replenishment of organics was studied at various stages of depletion of these compounds. In order to make the results as close to practical operating conditions as possible, the effect of replenishment was investigated also in the periods preceding and following the activated charcoal clean-up.
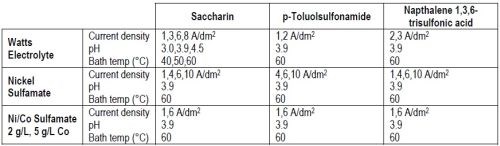
Table 1 presents an overview of the experimental conditions investigated. Because of space constraints, only a small sample of the results generated in the course of three years will be discussed.
Table 1 - Variables during the long-term experiments.
Evaluation methods
High-performance liquid chromatography
Applications of HPLC to the monitoring of organic additives have been reported in recent literature.10,12,13 In our experience, liquid chromatography is the only method capable of simultaneous determinations of trace levels (mg/L) of additives and of their decomposition products in the presence of much larger concentrations (g/L) of inorganic bath constituents. The chromatographic system* consisted of a quaternary gradient pump with a built-in sample loop injector and a programmable UV detector. Chromatographic columns were placed in a radial compression module. Additional experimental details are described in the captions of Fig. 2.
The leveling agent, butynediol, shown as a peak in Fig. 2, has not been used in our investigation series. Its presence in the chromatogram illustrates the usefulness of the multiple wavelength UV detection, as opposed to the more traditional single wavelength detection. Most chromatographers will be inclined to use the wavelength of 254 nm for the detection of an aromatic compound (e.g., saccharin), yet a lower wavelength yields a higher sensitivity for this compound and enables determination of an additional bath component which would otherwise remain undetected. Among the compounds that cannot be detected satisfactorily at 254 nm are the decomposition products of saccharin. With the newly introduced programmable and scanning UV detectors, chromatograms can be obtained at four or more wavelengths simultaneously after a single injection of a sample. These instruments eliminate the necessity of sequential injections and recordings for sample components with different UV absorption maxima.
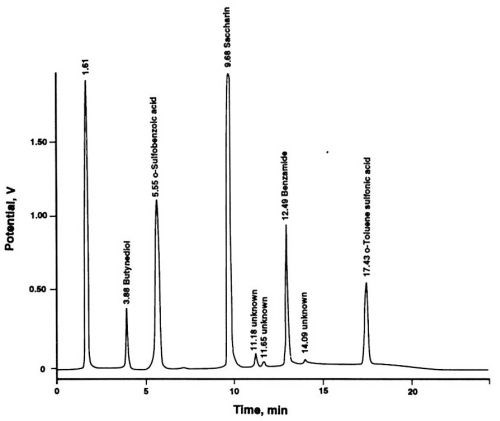
Figure 2 - Liquid chromatographic separation of saccharin and its decomposition products. Column: 5 μm; Eluent A: 10 mM aqueous NaH2PO4 adjusted to pH 2.4 by adding dilute phosphoric acid; Eluent B: HPLC-grade acetonitrile; Linear Gradient Elution: From 100% A, 0% B at the time of injection to 65% A, 35% B after 20 min; Flow rate: 2 mL/min; UV Detection: 195 nm (1.0 AUFS) from start to 5 min ; 220 nm (1.0 AUFS) from 5 to 12 min, 254 nm (0.75 AUFS) from 12 to 20 min; Sample: 50 μL of used Watts nickel electrolyte solution.
Ductility and tensile strength
Several methods for evaluation of the ductility of thin metallic films have been reviewed by Nakahara and Okinaka.14 A hydraulic device, based on the design by Deubel and Lurie and optimized by Rolff, was chosen for evaluation of ductility.15,16 By using a specialized arrangement included in the same instrument, it was also possible to measure and calculate tensile strength, according to an equation derived by Prater and Read:17
(1)
where Rm is the tensile strength, p the experimental pressure, a is nickel layer thickness, and R is the radius of a sphere obtained by extension of a nickel callotte generated during the ductility test.
Internal stress
Numerous methods of internal stress measurement have been described in the literature. Our evaluation was carried out with the help of an instrument designed by Dvorak, Prusek and Vrobel (IS Meter).18 A deformable coil of brass is immersed in the bath sample and connected as a cathode. Dimensional changes resulting from electrodeposition of nickel are evaluated with the help of inductivity measurements.
Vickers hardness
All measurements of hardness were made according to a procedure specified in ISO 4516-1980. A commercially available instrument** was utilized. The relative standard deviation was evaluated as about four percent, corresponding to 10 hardness units. The maximum pressure at the low values of layer thickness generated was determined as 0.49 N (50-g load).
Results and discussion
Short term experiments with newly prepared bath solutions
The main objective was to determine the role of typical organic additives in nickel plating solutions under conditions as realistic as possible. The value of pH, current density and throughput in A-hr, as well as the levels of the inorganic bath constituents, can be easily maintained and documented by the equipment and procedures available to the large majority of electroplaters. Correlations between these easy-to-maintain parameters and the quality of the plated nickel, such as the two examples shown in Figs. 3 and 4, are also readily available. For this reason, those parameters were kept constant and concentration was on organic stress-reducing additives. The parameters influencing the internal stress of electrodeposited nickel layers are as follows:
• current density at the cathode
• pH of the solution
• temperature of the bath
• chloride concentration
• choice of stress-reducing agent
• choice of leveling agent
• type of anode
• fineness of filtration
• impurities
• hydrodynamics
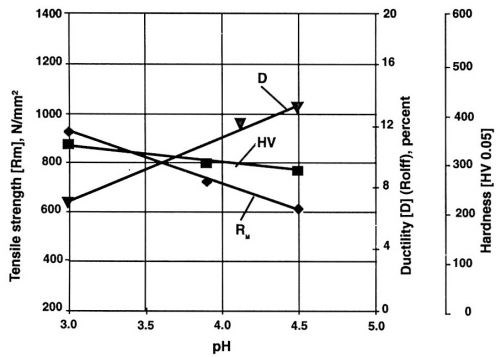
Figure 3 - Mechanical properties of nickel layers at different pH values of the electrolyte. Watts nickel bath, temperature 50°C, current density 1 A/dm2, saccharin concentration 100 mg/L.
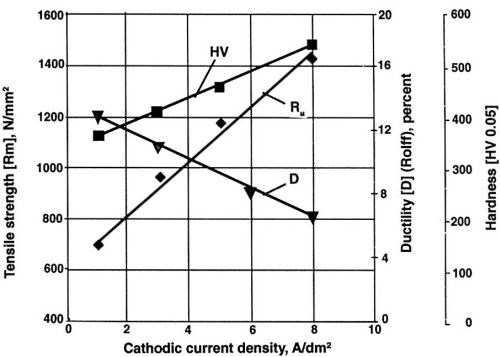
Figure 4 - Mechanical properties of nickel layers at different current densities during electrodeposition. Watts nickel bath, temperature 50°C, pH 3.9, saccharin concentration 100 mg/L.
Figure 5 illustrates the influence of the stress reducer saccharin on the value of internal stress in the Watts electrolyte. It is evident that without the addition of saccharin, nickel deposits are likely to exhibit rather high positive values of internal stress. In electroforming, however, desirable results are characterized by a relatively low or even negative value of this property (compressive stress). For this reason, saccharin is preferred for most applications.
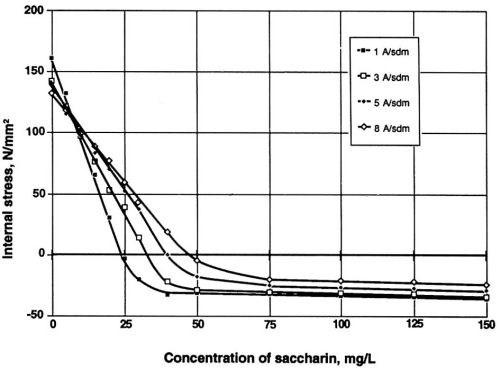
Figure 5 - Dependence of internal stress on the concentration of saccharin at four different cathodic densities. Watts nickel bath, temperature 50°C, pH 3.9.
Note the decreasing value of ductility with increase of current density (Fig. 4) and the lowest value of internal stress at one A/dm2 for saccharin (Fig. 5). Much higher concentration and a higher current density are required for similar results with TSA. With NTSA, even higher concentration and current density are needed to produce an internal stress-free nickel layer. As the internal stress generally increases in going from Watts bath to nickel or nickel-cobalt sulfamate, saccharin becomes the only additive known to reduce effectively the internal stress in all available compositions of nickel baths.
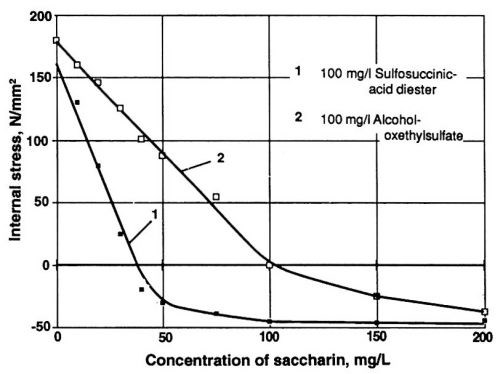
Figure 6 - Wetting agents influencing the stress reduction efficiency of saccharin.
Further, effective control of internal stress may be made even more difficult by the cross-influence of some organic wetting agents. As demonstrated in Fig. 6, alcohol oxethylsulfate inhibits to a significant degree the stress-reducing influence of saccharin. On the other hand, it is also possible to find wetting agents not interfering with stress reduction. Compare curve 1 in Fig. 6 with the one-A/dm2 curve of Fig. 5. Based on this observation, the sulfosuccinic acid diester was chosen as a wetting agent. Its concentration was maintained at a constant level and monitored with the help of the methylene blue photometric technique.19
The correlations just discussed, while quite useful in understanding the basic trends for a given bath type, still do not provide all the answers needed for successful long-term operation of nickel plating baths.
Long-term evaluation of nickel plating solutions
In the first of the long-term experiments, a bath sample was subjected to a long series of electrodeposition steps, while documenting the "bath age" in A-hr/L. All bath parameters were kept constant with the exception of saccharin concentration, which was allowed to decrease to zero (Fig. 7a). The nickel layer sample obtained after each of the electrodeposition steps was peeled off the polished stainless steel substrate and evaluated, using the procedures described in the Experimental Procedure section above. Results of these measurements are summarized in Fig. 7b. In order to estimate the rate of transfer of molecules of the stress-reducing agent and its decomposition products from the bath into the nickel layer (Coulombic efficiency), the sulfur and carbon content in the nickel layer samples after each electrodeposition step were also determined. For practical purposes, it is useful to identify four distinct working ranges in the life cycle of the Watts bath (Table 2).
Table 2 - Working ranges in the life cycle of the Watts bath.

If similar evaluations are carried out with saccharin-containing nickel and nickel-cobalt sulfamate baths, very similar diagrams, as in the case of the Watts bath sample, can be generated. Saccharin is evidently capable of achieving similarly high levels of negative internal stress in all three types of nickel electrolytes. A practical consequence is perhaps the observation that at comparable initial concentrations of saccharin, working range A is twice as long in nickel-cobalt sulfamate as in the Watts bath.
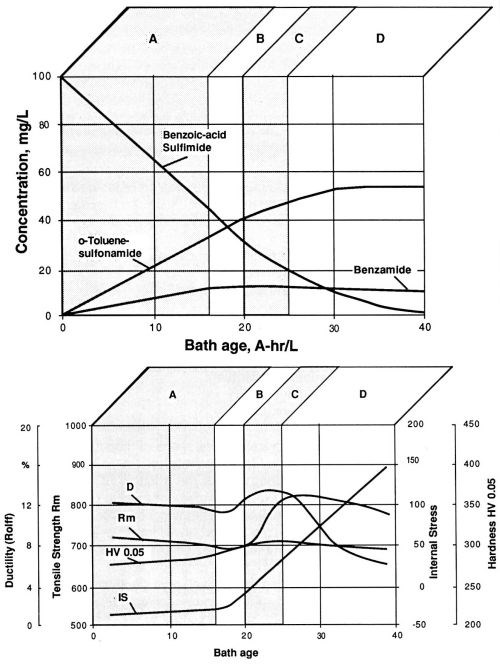
Figure 7 - Watts nickel bath, temperature 50°C, current density 1 A/dm2, pH 3.9; (a) decomposition of saccharin (benzoic acid sulfimide) and the build-up of corresponding decomposition products during electrolysis in a freshly prepared electrolyte solution; (b) fluctuation of the values of mechanical properties during use of a freshly prepared Watts nickel bath solution.
In the next step, the possibility of maintaining a desirable bath performance by replenishment of the depleted concentration of the stress-reducing agent was investigated. According to the results (Figs. 8a and 8b), saccharin replenishment would only in certain cases bring about return to working range A. During the experiment described in Figs. 8a and 8b, a properly chosen moment for saccharin replenishment leads to a considerable extension of working range A. Once working range B is reached, even readjustment of the saccharin concentrations to the initial level does not suffice to produce the conditions of working range A. Note that the working ranges as defined above are characterized more by the molar ratios of saccharin to its main decomposition products than by the saccharin concentration alone. Without monitoring the levels of saccharin and its decomposition products by liquid chromatography, choosing the proper timing for saccharin replenishment can be characterized as an extremely difficult, if not impossible, task.
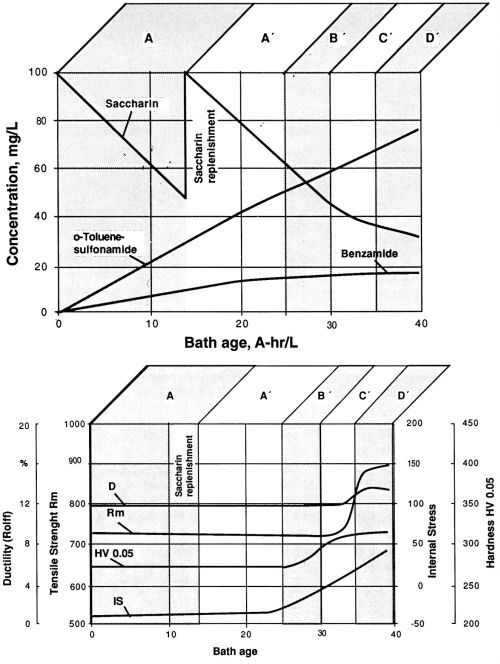
Figure 8 - Replenishment of saccharin after its concentration was reduced by electrolysis to 50%. Watts nickel bath, temperature 50°C, current density 1 A/dm2, pH 3.9; (a) saccharin and its decomposition products before and after replenishment; (b) fluctuation of the values of mechanical properties before and after replenishment of saccharin. Compare with Fig. 7.
Replenishment of the stress-reducing agents appears to be considerably easier with the other two compounds chosen. With TSA, working range A can be readjusted even after working range D of a Watts nickel bath has been reached. In the case of NTSA, we were able to return to the original stress plateau after a bath replenishment carried out in working range C. As already discussed, both TSA and NTSA do not produce any detectable decomposition products in the plating bath. One of the characteristics of such plating bath compositions is the absence of working range B. With depletion of the stress reducer, such baths change from working range A directly to working range C. It can be concluded that use of a less efficient stress reducer allows easier maintenance of the electrolytes and thus appears to be advantageous for some of the less difficult applications of nickel plating.
Analytical control of carbon treatment
Activated charcoal remains the most popular method of removal of organic compounds from spent plating solutions. Attraction between an organic molecule and a given type of charcoal can be caused by a wide range of mechanisms ranging from simple adsorption to some of the more permanent forms of attachment, such as, for example, chemisorption. In consequence, there are many varieties of activated charcoal offered to electroplaters. The essential differences are variations of pore size and distribution, as well as varying values of the internal surface.
The liquid chromatographic method originally developed for monitoring saccharin and its by-products in a nickel bath, can also be used for evaluation and analytical control of carbon treatment. Rates of removal of organics from the treated electrolytes are determined as a difference in concentrations, found by comparing the results obtained before and after addition of a known amount of activated carbon.
Efficiency of the charcoals for the removal of saccharin and its decomposition products from nickel electrolytes was evaluated first for each of those compounds alone. Results from these tests are compiled in Table 3.
Table 3 - Removal of saccharin and its by-products from nickel electrolytes.
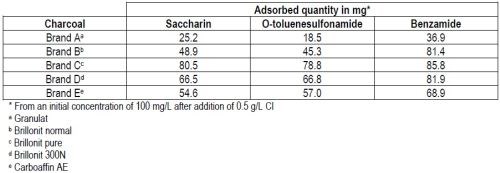
With all five brands of carbon tested, benzamide was more strongly adsorbed than the remaining two compounds. Contrary to original expectations, no correlation could be found between the sorption efficiency and the value of the internal surface as measured by the BET method (Brunauer, Emmett and Teller).
If mixtures of organic compounds are subjected to carbon treatment, the results may be influenced by mutual displacement phenomena. In varying degrees, we have observed similar behavior with the organic compounds under study exposed to the five brands of carbon. One brand*** gave the best results; for this reason, it was included in the final evaluation, during which the pretreatment concentrations of all three organic compounds were 100 mg/L and the addition of carbon varied from 0.5 to 3.5 g/L (Fig. 9). It can be concluded from the results shown in Figs. 7 and 8, that a certain minimal concentration of saccharin byproducts has no negative effects on the quality of the nickel layer. With this in mind, the necessary addition of activated charcoal*** can be estimated from Fig. 9 as approximately two g/L. After addition of this quantity of carbon, followed by filtration and readjustment of the saccharin level to 100 mg/L, the treated bath sample is able to perform in working range A for a length of time (A-hr/L) equal to that of a newly made-up solution.
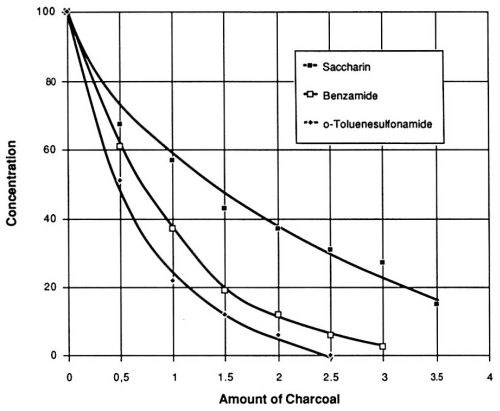
Figure 9 - Final concentrations of saccharin and its decomposition products at different levels of added activated charcoal. Watts nickel bath volume 1 L, temperature 50° C, pH 3.9.
Another striking feature of Fig. 9 is the faster rate of removal of the two decomposition products in comparison with saccharin. This observation led to another series of experiments with the objective of developing continuous removal of byproducts from a bath while maintaining a constant level of saccharin. The constant levels of this component could be achieved by a continuous displacement purification procedure.
The most promising version of this new technique involves continuous recycling of a nickel electrolyte through a bed of activated carbon.*** In the first stage of the procedure before actual use, the concentration of saccharin is repeatedly readjusted to 100 mg/L for as long as necessary to saturate the carbon material. The second stage of the procedure is represented by practical use of the solution for the nickel plating. During this second stage, recycling of the bath through the carbon bed continues. As the decomposition products are adsorbed on the carbon bed, saccharin is released into the plating solution. In so doing, the saccharin concentration in the bath is held fixed, while its decomposition products are constantly being removed from the bath.
With the current version of the method, exact prediction of the sulfur and carbon content in the nickel layers is still not possible. The bath samples can be kept in working range A for a much longer time (A-hr/L), however, than with the standard procedure allowing the saccharin products to accumulate. Exact prediction of the breakdown of the continuous displacement purification appears to be relatively difficult, especially with the different batches of carbon material in question. For this reason, close monitoring of the bath by HPLC during the second stage of the displacement purification was found very important in the long-term operation.
Conclusions
Reliable deposition of high-quality nickel layers requires continuous control of the key parameters. Applications of HPLC as suitable methods for monitoring organic additives and their decomposition or reaction products in nickel electrolytes have been developed. By such means, correlations between the organic components of nickel plating baths and selected layer properties can be demonstrated.
References
1. L. Winkler, Metalloberfläche, 21, 225, 261-267 (1967).
2. W.H. Safranek, The Properties of Electrodeposited Metals and Alloys, Am. Electroplaters and Surf. Finishers Soc, Orlando, FL, 1986.
3. R. Suchentrunk and O. Tuscher, Galvanotechnik, 70, 1178 (1979).
4. W.R. Wearmouth and K.C. Belt, Trans. Inst. Metal Finish., 52, 114 (1974).
5. R.J. Walter, Plating and Surface Finishing, 67 (7), 46 (1980).
6. J. Dubsky and P. Kozak, Metalloberfläche, 24, 423 (1970).
7. W. Strauss, Tenside, 3, 144 (1966).
8. W. Immel, Galvanotechnik, 67, 459 (1976).
9. K.J. Zerweck, J. Böcker and T. Bolch, Proc. Interfinish 1980, Kyoto, Japan (1980).
10. P. Jandik, A.L. Heckenberg and R.L. Lancione, Proc. 2nd AESF Analytical Methods Symposium, Orlando, FL, (1988).
11. Final Report (zu II D1 -Ze 193/2-3), Fraunhofer Institute for Manufacturing, Engineering and Automation, Stuttgart, Germany, 1984.
12. R.L. Lancione, A.L. Heckenberg and P. Jandik, Proc. SUR/FIN 1987, AESF, Orlando, FL (1987).
13. P. Jandik, W.R. Jones, B.J. Wildman, J. Krol and A.L. Heckenberg, Proc. SUR/FIN 1989, AESF, Orlando, FL (1989).
14. S. Nakahara, Y. Okinaka and D.R. Turner, J. Testing and Evaluation, 5, 178 (1977).
15. J.M. Deubel and G.R. Lurie, Plating, 60, 715 (1973).
16. R. Rolff, Metalloberfläche, 30, 650 (1976).
17. T.A. Prater and H.J. Read, Plating, 36, 1221 (1949).
18. A. Dvorak, J. Prusek and L. Vrobel, Metalloberfläche, 27, 284 (1973).
19. Deutsche Einheitsverfahren zur Wasser-, Abwasser-und Schaumuntersuchung, H 23: Bestimmung von Detergentien, 6th Ed., Verlag Chemie, Weinheim, 1971.
About the Authors
Dr. Jurgen W. Böcker is supervisor of thin film processes at IBM Sindelfingen in the multilayer ceramics division. He received a MS in chemistry from the University of Hamburg and a PhD in engineering from the University of Stuttgart. He is a lecturer on surface technology at the Fachhochschule Aalen, where he also teaches instrumental analytical chemistry.
Thomas Bolch is section manager of electroplating processes at the Fraunhofer Institute of Manufacturing, Engineering and Automation (IPA), Stuttgart, Germany. In 1989, he won the Heinz Leuze Award from the German Electroplaters and Surface Finishers Society (DGO), and in 1990 the Fraunhofer Award - both with co-author Gemmler - for their work on electrochemical decomposition of chlorinated hydrocarbons. He holds a diploma from the Fachhochschule (Technical College), Aalen, Germany.
Dr. Armin Gemmler is responsible for development and analysis of electroless and electroplating processes at the Fraunhofer IPA, where current topics include investigation of integration of knowledge into computerized expert systems. He holds a Ph.D. in inorganic chemistry from the University of Stuttgart.
Dr. Petr Jandik is R&D manager for the Waters Chromatography Division of Millipore Corp. For seven years, he worked in the R&D Department of Siemens AG (Munich) on the manufacture of printed circuit boards. He obtained a M.S. in analytical chemistry from Clarkson University and a Ph.D. in inorganic chemistry from the Technical University, Munich, Germany.
Related Content
Innovation in Plating on Plastic
Plating on advanced plastics solution offers improved adhesion, temperature resistance and cost savings.
Read MoreHow to Choose Between Sulfate and Chloride-Based Trivalent Chromium
There are several factors to consider when choosing between sulfate and chloride-based baths for trivalent chromium plating. Mark Schario of Columbia Chemical discusses the differences and what platers should keep in mind when evaluating options.
Read MoreSuccessful South African Plater Beating the Odds
Remaining focused on quality and reliability, Team Plating Works stays profitable in a volatile and challenging economy.
Read MoreTroubleshooting Alkaline Zinc
One of the most common problems that can arise when plating with alkaline zinc is an imbalance of brightener in the solution. In this helpful Ask the Expert article, Chad Murphy of Columbia Chemical discusses how different zinc metal concentrations and brightener concentrations can impact efficiency.
Read MoreRead Next
Education Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read More











.jpg;maxWidth=300;quality=90)









