Using Ion Exchange in Trivalent Chromium Systems
Shane Moore of Pavco discusses the role of ion exchange technology in trivalent chromium systems for maintaining a consistent deposit.
Q: My chemical supplier recommended using ion exchange technology to remove metallic contamination from my trivalent chromium solution before it begins to accumulate. What information do I need to install this correctly?
A: The topic of ion exchange is often being discussed as more and more applicators make the switch from hexavalent to trivalent chromium. The trivalent chromium systems are much more sensitive to metallic contamination than hexavalent, so a method of removal is necessary. Otherwise, loss of production may occur during First, you must determine the type and quantity of resin needed for your application. For removal of metallic contamination from a trivalent chromium solution, a cationic (preferably chelating) resin should be chosen. The resin must be engineered to selectively remove the most common elements introduced to a trivalent chromium solution – copper, zinc, nickel and iron. The quantity of resin needed will depend on the substrates processed, as most resins have a stronger affinity for some metals, and weaker affinity for others. Another variable to be considered during this calculation is the fact that certain metals are more damaging at lower concentrations than others. For example, copper contamination can begin causing issues at 3-4 ppm, while nickel can accumulate to over 50 ppm before seeing any issues arise. Most of the ion exchange resins used in our industry have the strongest affinity for copper, so it is removed soon after being introduced to the solution. These common resins usually have a weaker affinity for zinc, so the resin needs more contact time with the solution to remove the zinc. If an applicator processes a high percentage of zinc diecast, it may benefit them to use a larger quantity of resin, so there is more active surface area for its removal. If the resin is chelated, it contains functional ligands to assist in binding the metals to the resin for more efficient removal. Typically, the rule of thumb is to incorporate 1 cubic foot of resin per 1000 gallons of plating solution. For applicators processing brass and zinc diecast, 2 cubic feet/1,000 gals would be preferred. It is important to note that the resin should be in place before production begins. Otherwise, loss of production may occur during mass removal of accumulated metallic contamination, instead of the preferred continuous removal.
The resin chamber should be filled to 50% capacity to allow for resin enlargement or swelling. The resin is then rinsed well to remove the residual dyes, etc, then it must be activated. If the resin operates most efficiently as a “Sodium-based” bed, then the resin must be activated with dilute sodium hydroxide. The most common resins used in our industry are “Hydrogen-based” beds, so must be activated with a dilute acid, such as sulfuric acid for sulfate-based trivalent chromium systems, or hydrochloric acid for chloride-based systems. Using the hydrogen-based resin as an example, the activation step causes the negatively charged active sites on the resin to accept the hydrogen atom which is then positively charged and is ready to be exchanged for metallic contamination in order of its affinity rating. The resin is then rinsed until the pH is close to operation parameters. The resin is then ready to be placed into operation.
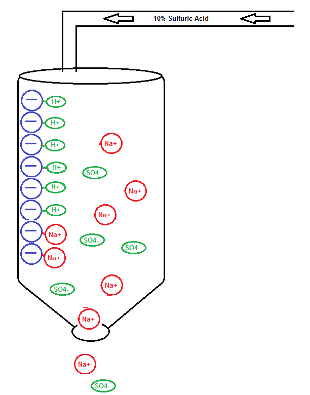
Activation Procedure (Converts to H+ based bed)
Photo Credit: All images courtesy of Pavco
Initially, the flow rate should be set at 2-3 gals per minute per cubic foot. It can be adjusted to each specific application as data and experience is generated. The plating solution must be filtered through a 5-micron guard filter before entering the chamber, to ensure no particles enter the chamber and damage the resin. Once activated, the resin should never be allowed to completely dry out, as this will also damage the resin and compromise its performance.
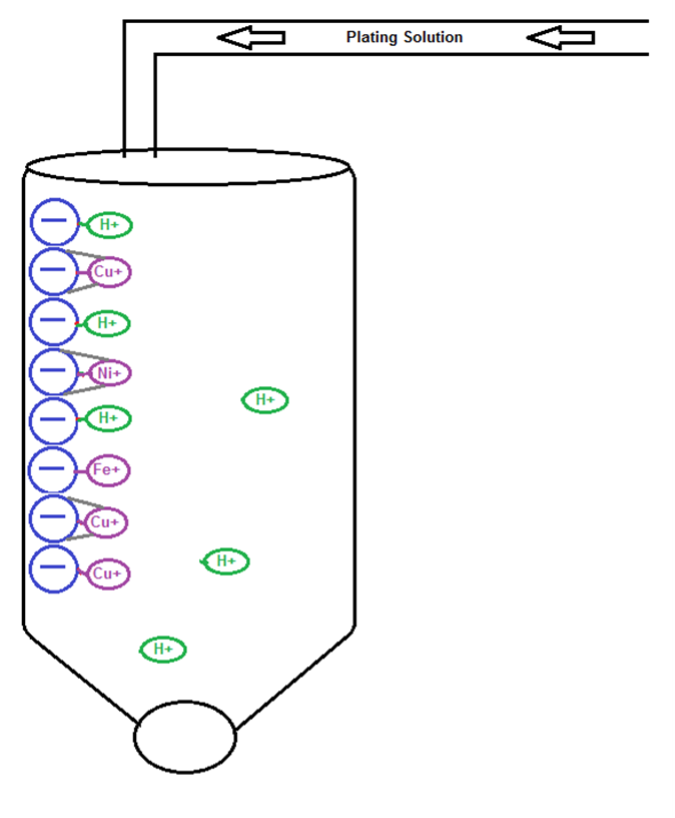
Hydrogen molecules are displaced by metallic contamination. Ionic bonds and ligands from chelating resin retain metals until regeneration.
Once the resin has reached saturation, it must be regenerated. This is evident by taking a sample of the plating solution and another directly from the resin discharge and analyzing the metals in both. As the metallic concentrations from the resin discharge increase to match those in the tank, the resin must be taken out of operation and regenerated. The first step in regeneration is the backwash step. This involves flowing DI water from the bottom of the chamber and exiting the top of the chamber, so the resin is lifted during this rinse stage, then settles back to the bottom of the chamber. The next step is the ammonium hydroxide regeneration. Dilute ammonium hydroxide is either flowed through the chamber and back to its holding tank, or the chamber is filled with dilute ammonium hydroxide, allowed to soak for an hour, then sent to waste treatment. This step may need to be repeated 1-2 times to ensure complete copper removal. This step will remove all copper ions, and some of the other metallic elements. After this step is complete, the resin must be rinsed with DI water until the pH is no longer alkaline.
The next step in regeneration is the acid step. Dilute sulfuric acid (or hydrochloric acid in chloride-based systems) is either flowed through the chamber and back to its holding tank, or the chamber is filled with the dilute acid and allowed to soak for an hour, then sent to waste treatment. This step removes all of the other metallic elements and places the bed back into its hydrogen phase. The resin is then rinsed well with DI water until the pH is near operating parameters. The resin can then be placed back into service. If care is taken to prevent particles from entering the chamber, and hydration is always maintained, the resin will last for many regenerations.
The use of ion exchange technology in trivalent chromium systems is of key importance in maintaining a consistent deposit. The constant purification of the solution will eliminate the co-deposition of these alloying metals, which negatively affect appearance and corrosion protection.
About the Author

Shane Moore
Shane Moore is a technical service engineer and the decorative team manager for Pavco Inc. Visit pavco.com.
Related Content
Liquid Chrome Vs. Chromic Acid Flake
Contemplating how to continue offering chromic acid services in an increasingly stringent regulatory world? Liquid chrome products may be the solution you’re looking for.
Read MoreA Chromium Plating Overview
An overview of decorative and hard chromium electroplating processes.
Read MoreSuccessful South African Plater Beating the Odds
Remaining focused on quality and reliability, Team Plating Works stays profitable in a volatile and challenging economy.
Read MoreProducts Finishing Reveals 2023 Qualifying Top Shops
Each year PF conducts its Top Shops Benchmarking Survey, offering shops a tool to better understand their overall performance in the industry. The program also recognizes shops that meet a set of criteria to qualify as Top Shops.
Read MoreRead Next
Episode 5: Transitioning Hexavalent Chromium to Trivalent, Part 1
In this episode of On the Line, Products Finishing editor-in-chief Scott Francis sits down with Mark Schario, executive vice president with Columbia Chemical to hear about the latest innovations in trivalent chromium plating technology.
Read MoreMaking Chrome Safe
A non-aqueous hard chrome alternative offers safe and environmentally sustainable functional and decorative chrome coatings.
Read MoreSwitching from Decorative Hexavalent Chromium to Trivalent
Pavco decorative team manager, Shane Moore, discusses the considerations you should keep in mind when contemplating switching from decorative hexavalent chromium to trivalent.
Read More





















