White Bronze, Copper-Tin-Zinc Tri-metal: Expanding Applications and New Developments in a Changing Landscape
This paper deals with the renewed interest in applications for white bronze tri-metal (Cu-Sn-Zn alloy).
by Richard E DePoto and Al Gruenwald C., Uyemura & Co. Ltd., & Joerg Weber and Klaus Leyendecker, Umicore Galvanotechnik GmbH
ABSTRACT
This paper deals with the renewed interest in applications for white bronze tri-metal (Cu-Sn-Zn alloy). The increased interest is driven by several factors. Continually increasing electronic frequency demands and tightening of boundary bandwidths requires components which are corrosion resistant, non-magnetic and have higher hardness properties. The cost of precious metals has risen sharply, with silver consistently selling at over $25 per ounce. Recent research and applications developments have improved process control, ease of analytical measurement and resulting performance of tri-metal alloy electroplating. New proprietary formulation-based enhancements including a high speed version, have been introduced to expand the product usage and applications. Tri-metal “white bronze” chemical processes have historically been difficult to control and too often produced a less desirable and less capable alloy than was required. Recent applications development has improved these chemical systems, determined the most critical control parameters and control points, and standardized the preferred analytical techniques for precise alloy control. Specifically, these improved techniques allow for a preferred alloy to be plated consistently, resulting in higher performance and expanded applications in the electronic industry.
Keywords: tri-metal, copper-tin-zinc, alloy plating, electronics, white bronze
Introduction
The plating of copper-tin alloys has been done for many years and is widely used for a variety of applications. The most common processes are the plating of brass for decorative purposes and the plating of copper-tin deposits for electronic components. Recently there has been an increasing requirement for high performance and specialty electrolytic deposited layers. For example, decorative layers and electronics applications now must fulfill higher technical requirements for corrosion resistance, deposit hardness and wear resistance.
The properties of single-metal deposits are fairly stable and can only be slightly enhanced. Process enhancement emphasis is restricted to improved brightener and leveling additives which improve metallurgical properties such as ductility, elongation and overall process stability. By depositing two or more metals simultaneously to form an alloy coating, we can produce properties that are not possible with single metal systems. The properties and performance can be varied by considering an unlimited number of alloying elements and alloy compositions. Therefore the properties of the deposits can be tailored to fulfill specific requirements and customized applications.
A perfect example of this approach is the plating of tin alloys, copper-tin alloys and specifically copper-tin-zinc alloys. Copper-tin alloys invented over 40 years ago are now being refined and used in a wide variety of applications from jewelry and architecture to medical and electronic connector parts. In most manufacturing process sequences, copper-tin alloys are plated over acid copper deposits, which tend to level the underlying deposit and increase the alloy coating adhesion
White bronze is not actually bronze. It is an alloy consisting of a combination of copper, tin and zinc. Tri-metal alloys are white in color, similar to bright nickel, silver or rhodium and are extremely resistant to tarnish and corrosion. The alloy range is centered around 55% copper, 30% tin and 15% zinc.
The impetus to develop processes for this deposit alloy initially came from the "nickel-free" legislation first introduced about 15 years ago and was intended to address costume jewelry. Nickel-free requirements have now crossed over into clothing fasteners, car interior trim and numerous commercial and consumer electronic applications. With nickel being banned from any potential skin contact application in the European Union, tri-metal is considered the preferred, practical and safe alternative for items that can come into contact with the skin. Staggeringly, 15% of the population today is estimated to have an allergic reaction to nickel as opposed to only 10% in the 1980s suggesting nickel-related allergies appear to be on the rise. Tri-metal white bronze eliminates this problem and has been proven to be a cost effective and safe alternative.
Because of its appearance and chemical properties - being highly resistant to corrosion and wear, being solderable, non-magnetic, smooth and non-porous - tri-metal is an ideal substitute for nickel and silver for high frequency RF connector and other electronic applications. The bright white finish of tri-metal plating can also be used as an undercoat with palladium, palladium-nickel, silver or gold products or as a topcoat with those finishes. Tri-metal plating creates a non-toxic, non-magnetic deposit that is highly resistant to corrosion. This metal finishing application and deposit has low porosity and a low coefficient of friction. Lead-free tri-metal plating is outstanding for solder applications.
This paper focuses on our work on a recent advance in tri-metal plating, a proprietary copper-tin-zinc alloy** which meets the requirements for a robust nickel substitute, among many other advantages. We discuss here both operational properties as well as several of the performance properties required in the many applications noted above.
Tri-metal chemistry
The plating process is a cyanide-based plating solution, with organic additives that give excellent brightness and some degree of leveling, even with thicknesses of only 2 or 3 µm. The chemistry operates in either rack or barrel mode and is suited to deposition on steel, brass, copper or zinc diecast basis material. An important feature of tri-metal chemistry is the throwing power of the process. The process is sufficiently capable to plate the same thickness inside a zip fastener slider as the outside of the fastener. Plating can be accomplished even in a barrel application. This throwing power feature, combined with electrical wear resistance, non-tarnishing properties and low cost has made white bronze popular with companies who previously would probably have used silver with an anti-tarnish coating. The process and the chemistry is relatively straightforward, well understood and uses standard analytical capabilities. Baths routinely have a very long life. In fact, depending on the manufacturer, many bath solutions in use today are seven or eight years old and still performing well.
One of the few procedural adjustments recommended when running a tri-metal production process is the frequency of analytical measurement as compared to single metal systems. Since our goal is to control the plated alloy ratio tightly, keeping the corresponding ratio of the individual metal concentrations consistent is a critical characteristic. As one plates the target alloy, the metal concentrations will drop accordingly and keep the metal ratio in balance. Although this is a distinct advantage, slight adjustments are always part of well-run processes. Metals are added on a prescribed schedule based on ampere-hours of plating and are easy to calculate and very predictable. Specific metal chelaters and complexers such as hydroxide, cyanide and organic additives, are also consumed according to ampere-hours and time-related decomposition and have to be analyzed more frequently. Typical chemical additions and analyses are made on a shift basis and at startup but soon become predictable and well-understood methods.
Process sequence
The basic process sequence is depicted in Fig. 1 below and is very similar to single metal plating processes that exist in any plating facility. Pre-treatment steps are comprised of a soak cleaner followed by a standard reverse electrocleaning step. This step is followed by an acid activation before a standard cyanide copper strike. In some processes, acid copper is used because of its higher leveling capability and the fact that tri-metal plating tends to duplicate the underlying plated surfaces more closely.
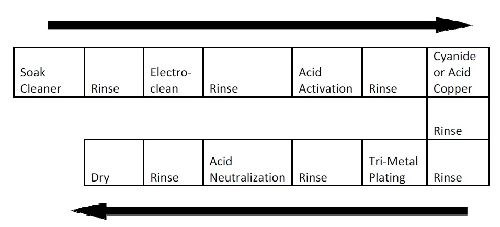
Figure 1 - Typical copper-tin-zinc alloy plating process flow.
The tri-metal plating rate of approximately 22.9 nm/min (0.9 µ-in./min) requires a 15-min cycle at 0.5 A/dm2 (5.0 A/ft2) to deposit the preferred 3.0 µm (120 µ-in.) of alloy. Three to four tri-metal plating cells per bath normally balance a full production line, although that depends greatly on the thickness of underlying copper that is being plated.
The deposits of Cu-Sn and Cu-Sn-Zn alloy processes are achieved with an alkaline solution containing cyanide and hydroxide as metal chelators. A proprietary brightener system helps to compensate and buffer any variation in the concentrations of the major components of the bath. This helps to provide and maintain a specific alloy deposit composition. The target concentrations of the components and the wide operating window for our proprietary additive system are shown in Fig. 2.
One of the most important parameters to target and control is the plating current density. This is because the alloy ratio is highly dependent upon current density and will favor a copper-rich alloy at high current densities and a tin-rich alloy at low current densities. The ideal (largest operating window) current density for the proprietary process is centered at 0.5 A/dm2 (5.0 A/ft2). However, the additive system was optimized to compensate and plate a tight percentage alloy over the range of 0.2-0.8 A/dm2 (2.0-8.0 A/ft2). The value of this wide window is considerable. Within a single part and location on the rack, current densities can have a fairly high variability. The specific part design, rectifier sensitivity, number of anodes, anode spacing, size and distance from the plated part all have profound effects on localized part current density. The capability of the additive system to buffer and compensate for these variations is critical in holding the target alloy and the resulting color and/or brightness of the part.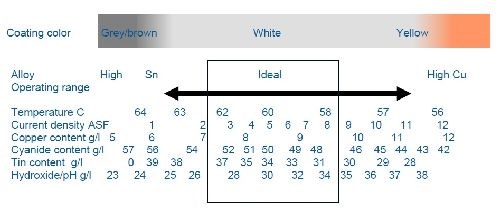
Figure 2 - Influences of electrolyte parameters.
Alloy composition capability as-plated
Improved processes have been developed and are available for both rack and barrel plating which have excellent capabilities in holding tight alloy compositions. Figure 3 shows the alloy variability of the plated Cu-Sn-Zn alloy on a family of brass connector parts processed in a large 2000-L production bath. The alloy content was measured using an x-ray fluorescence unit manufactured by Fischer Scientific. The alloy composition of the brass parts was held to a tight tolerance centered at 54% copper, 33% tin and 13% zinc over a month-long continuous running period.
Based on the month-long period of time the bath demonstrated excellent capability to hold part alloy composition. The bath experienced all the typical aspects of process variation that a plating bath would normally experience, including chemical laboratory analysis, subsequent replenishment as well as weekend shutdowns and startups. These new alkaline and cyanide containing electrolytes combine with the newer additive buffering systems to deliver superior alloy composition control, stability, thickness uniformity and performance in comparison to weak acid electrolytes that were available in the past.
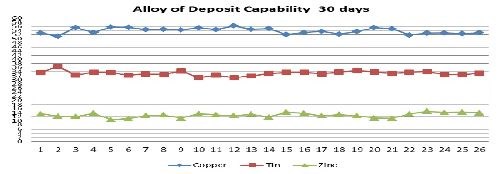
Figure 3 - Deposited alloy composition on brass connectors over a 30-day period (2,000-L production tank at the Unimetal, Naugatuck, Conn., facility.
Thickness uniformity and distribution
The tri-metal coatings are characterized by excellent metal thickness distribution and, depending on the type of bath, a high tarnish resistance, leveling, excellent abrasion resistance, corrosion resistance and good soldering properties. A comparison of the thickness distribution, using a Haring-Blum cell to determine throwing power, for the proprietary tri-metal bath is shown in Fig. 4 and is compared to single plated metals. From the graph, the thickness distribution for the Cu-Sn-Zn alloy is nearly equal to the thickness distribution for the individual metals of copper, tin and zinc. Tri-metal thickness distribution is much improved over bright nickel, chromium, acid copper and acid zinc single metal deposits in thickness variation and uniformity.
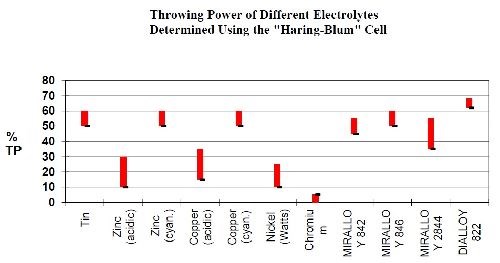
Figure 4 - Comparison of thickness distribution for common metal types to proprietary copper-tin-zinc alloys.
Table 1 shows thickness variation comparing tri-metal to other plated metals, including bright nickel, nickel-phosphorus and tin on a plated connector. Thickness uniformity ranges of less than 25% are very typical of well-engineered tri-metal processes and have proven to be a significant advantage in production flexibility and efficiency. Single metal system thickness variation on the same connector plated with bright nickel resulted in thickness variation five times the tri-metal level. This capability allows parts to be positioned with close spacing, even shadowing one another. This improves efficiency, thru-put capacity and minimizes tank volumes that are required. From a functional aspect, excellent thickness uniformity avoids overplating, in turn saving material cost. Additionally the process is able to hold tolerances required for threaded and tight fitting connectors.
Table 1 - Comparison of thickness distribution in µm of proprietary copper-tin-zinc to common metal types on standard connectors (Haring-Blum cell data).
Standard Connectors - Thickness Distribution in μm | |||||
Connector Used | Cu-Sn-Zn | NiP (P>11%) | Nickel | Bright Tin | Au-Co on NiP (P>11%) |
3.13 | 5.82 | 5.26 | 3.64 | 0.24 | |
2.88 | 3.92 | 3.34 | 3.06 | 0.23 | |
2.76 | 2.74 | 2.07 | 1.94 | 0.21 | |
2.56 | 2.76 | 1.52 | 2.52 | 0.21 | |
2.60 | 2.86 | 1.52 | 1.85 | 0.20 | |
2.91 | 3.21 | 1.46 | 2.28 | 0.21 | |
3.12 | 4.05 | 2.72 | 2.81 | 0.22 | |
3.41 | 6.42 | 9.75 | 4.06 | 0.20 | |
Alloy composition options
Depending on the desired performance, corresponding alloy compositions of either white-colored (lower copper) or yellow-colored (higher copper) deposits can be electroplated. High corrosion resistant white alloy deposits target 50-55% copper, 30-35% tin and 10-15% zinc. Yellow alloy coatings have a much higher copper content. A typical composition of a yellow deposit is 80% copper, 17.5% tin and 2.5% zinc. Typical alloy compositions are shown in Fig. 5.
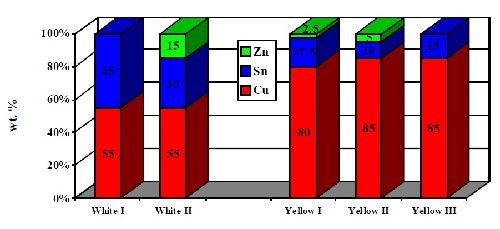
Figure 5 - Typical alloy compositions of Cu-Sn and Cu-Sn-Zn deposits.
Ongoing research and development of copper-tin alloys has resulted in a family of proprietary alloy processes which are shown in Table 2. Distinct alloys are listed and preferred applications, coating technique, properties (brightness / leveling) and maximum thicknesses are shown.
Table 2 - Typical alloy compositions and application data for proprietary Cu-Sn and Cu-Sn-Zn deposits.
Coating Color: White | ||||||||||
Product | Coating | Application | Alloy Composition (wt%) | Leveling | Max. Coating Thickness (μm) | |||||
Miralloy® | Rack | Barrel | Technical | Decorative | Cu | Sn | Zn | Brightness | Leveling | Depending on Substrate |
841 | x |
|
| x | 55 | 39 | 5 | retaining | x | 5 |
844 |
| x | x | x | 55 | 45 | --- | retaining |
| 5 |
2840 | x |
|
| x | 53 | 40 | 7 | retaining |
| 0.3 |
2841 | (x) | x | x | x | 52 | 40 | 8 | retaining | slight | 5 |
2844 | (x) | x | x | x | 55 | 30 | 15 | retaining |
| 5 |
2844E | (x) | x | x | x | 55 | 30 | 15 | retaining |
| 5 |
2850 | x | x |
| x | 50 | 40 | 10 | giving | slight | 15 |
2851 | x | (x) | x |
| 51 | 33 | 16 | giving | slight | 15 |
2852 | (x) | x | x |
| 50 | 41 | 9 | giving | slight | 15 |
Coating Color: Yellow | ||||||||||
Product | Coating | Application | Alloy Composition (wt%) | Leveling | Max. Coating Thickness (μm) | |||||
Miralloy® | Rack | Barrel | Technical | Decorative | Cu | Sn | Zn | Brightness | Leveling | Depending on Substrate |
846S | x |
|
| x | 75-80 | 13-19 | 5-8 |
| x | 20 |
3849 | x |
| x |
| 85 | 15 | --- | semi |
| 1000 |
847 |
| x |
| x | 80 | 17.5 | 2.5 |
| x | 20 |
2847 |
| x | x | x | 85 | 10 | 5 |
| slight | 50 |
848 |
| x | x |
| 80 | 17.5 | 2.5 | semi |
| 1000 |
Each alloy composition is achieved by using its distinct plating process. The reason for the varying parameters is to achieve the largest operating window and avoid large deviations of the alloy composition in the daily operation. The next generation formulations of tri-metal copper-tin Miralloy® alloys are denoted by 2850 and 2851 and can be electroplated at higher current densities and at higher maximum thicknesses.
Deposit properties
With regard to the brightness and leveling of the coatings, different degrees of leveling can be achieved. White deposits typically have brightness and retain the character of the underlying basis metal, which is usually electrodeposited copper. Brighter deposits are seen when using bright acid copper, which has better leveling than cyanide copper. Yellow layers are bright and level with an even lower porosity, and certain applications combine the leveling advantages of a yellow deposit with a white bronze overplate.
Applicable thicknesses up to 3.0 µm (120 µ-in.) for white deposits and 5.0 µm (200 µ-in.) for yellow high copper-bearing deposits are typical. Both deposits can be used as intermediate layers, e.g., in combination with precious metals over tri-metal deposits. However, the mirror white copper-tin-zinc layers, with their higher contents of tin, are predominantly used as final layers. This is based on their appealing bright white color and excellent tarnish/corrosion resistance. A long-standing application is the plating of zippers, snaps and buttons with a white tin-alloy as the final finish. These deposits replace the potentially allergenic nickel deposits that were previously in use.
Hardness and wear resistance
The copper-tin-zinc layers have a finely crystalline and homogeneous structure. The coating hardness of the white alloy at 550 VHN is between the hardness values of matte and bright nickel. Compared to tin and zinc, the Cu-Sn-Zn deposits show an improved hardness, as seen in Fig. 6. At 550 VHN, white Cu-Sn-Zn deposits exhibit a hardness close to that of a bright nickel deposit and significantly higher than copper, brass or tin or silver deposits. Increasing the copper content decreases the hardness proportionally with 350 VHN being typical at 80% copper. Improved hardness is one of the most important advantages of the alloy and has expanded its substitution for silver in both electronics and jewelry applications.
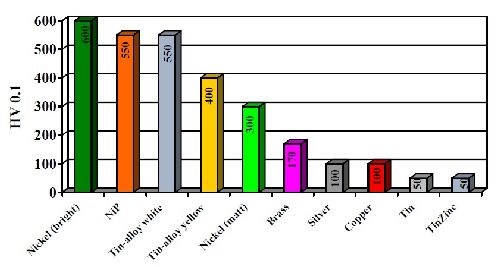
Figure 6 - Hardness of deposits of copper-tin alloys compared to single metals.
Tri-metal deposits have excellent abrasion resistance as compared to tin, zinc, silver and copper. The wear resistance of Cu-Sn-Zn deposits was measured and compared as shown in Fig. 7. The wear resistance testing was done using the Bosch-Weinmann Emery paper method (Swiss standard 6/0) / 300 g wt. The method measures the mass (mg) removal which occurs when a 300-g block laminated with Emery paper is slid across the plated deposit for 1000 strokes. A comparison to nickel, brass and silver is shown for reference, with white tri-metal positioned between nickel and brass and well ahead of silver.
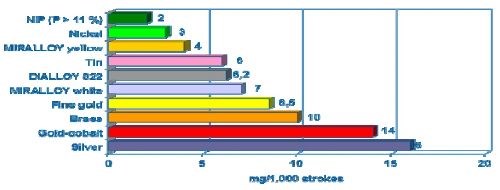
Figure 7 - Wear resistance of copper-tin alloys compared to single metals (Bosch-Weinmann emery paper (Swiss standard 6/0), 300 g wt).
The test was repeated using a Taber abrasion test technique, which is very similar in concept to the Bosch-Weimann test. The results showed very similar wear indexes for the deposits, as seen in Fig. 8. Tri-metal wear resistance was far superior to silver but not as good as bright nickel.
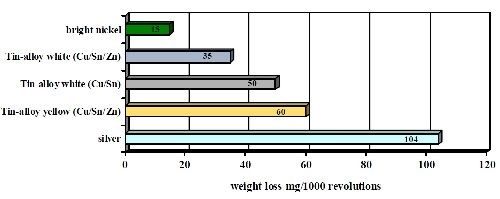
Figure 8 - Abrasion tests of copper-tin alloys compared to bright nickel and silver deposits (Taber abrader test wt. loss in mg/1000 revolutions).
Corrosion and oxidation resistance
As with casted copper-tin (bronze) alloys, plated copper-tin-(zinc) deposits are generally known to withstand corrosion quite well. By varying the ratio between tin and copper, the contact resistance, corrosion resistance and volume resistivity can be influenced and optimized. The results of a comprehensive battery of aging tests are shown in Fig. 9 comparing changes in the volume resistance of tri-metal compared to bright nickel. The environments included exposure to a quick temperature change, salt spray, mixed gas, high temperature, damp heat and a sulfur-containing atmosphere. The data shows that tri-metal equals or outperforms bright nickel coatings in every test and environment that was evaluated. In the sulfur-containing atmosphere, the tri-metal coating was particularly effective versus bright nickel deposits.
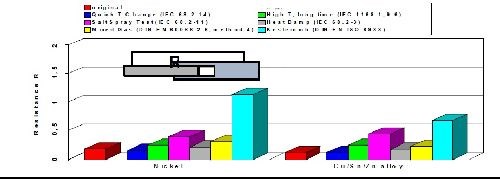
Figure 9 - Volume resistance of copper-tin-zinc versus bright nickel.
Figure 10 shows the result of a corrosion test under sulfur-containing atmosphere (Kesternich Test, according to DIN 50018-KFW 0.2S). The test results document the superior corrosion resistance of copper-tin-alloys as compared to nickel deposits on different basis metals including iron, brass and copper. By comparing results from different basis materials (copper, brass and iron), the test also evaluates the preferred basis metal application for the plating of tin-alloys, namely the plating on copper and copper-alloys.
In addition, the deposit thickness was also varied from 0.5 to 3.0 µm (20 to 120 µ-in.) and showed good correlation of corrosion protection and deposit thickness. This test is helpful in specifying minimum thicknesses for acceptable corrosion protection, whereby the tri-metal deposit has been appropriately standardized at 3.0 μm (120 μ-in.).
The higher electrochemical potential of the tin-alloy compared to the one of iron and the large gap between both potentials can cause anodic attack of iron. If iron is to be plated, the alloy composition should be changed to a copper-tin deposit (Fig. 10).
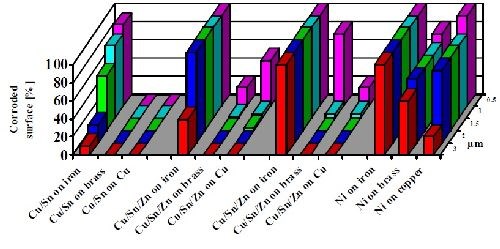
Figure 10 - Corrosion test results for tin alloy and nickel plated coatings on different basis materials in a sulfur-containing atmosphere (Kesternich Test/DIN 50018-KFW 0.2S). Variation of basis material and thickness: 0.5-3.0 µm (20-120 µ-in.).
Copper-tin-zinc and solderability
Tri-metal coatings are easy to plate over and, in combination with activated fluxes, they show good solderability and weldability. The excellent anti-tarnishing properties and good corrosion resistance of the Cu-Sn-Zn layers more than compensate for the slightly higher contact resistance versus silver. Most important, the tri-metal coatings ensure an acceptable volume resistivity, even in an atmosphere containing high humidity, high heat and sulfur, thus reducing component failure rate and making Cu-Sn-Zn the preferred overplate alloy.
Functional applications of Cu-Sn and Cu-Sn-Zn deposits
Tri-metal alloy finishes represent a cost effective and dramatic improvement over previous finishes of nickel, silver and stainless steel in RF connectors, filters and components applications. These applications frequently are exposed to highly corrosive environments where oxidation and tarnishing can be a detriment. Silver, which is the Standard Process of Record (POR) does have excellent electrical properties but tends to tarnish and breaks down, potentially allowing copper-tin-zinc to surpass silver as the POR for RF connector applications. Tri-metal copper-tin zinc is able to provide better throwing power and improved electrical performance versus both bright and matte nickel, based on its diamagnetic properties, lower permeability, better screening effects and lower intermodulation distortion (RF-applications). In addition, these electrical performance advantages are gained without a significant difference in hardness, wear and abrasion characteristics as compared to nickel.
The higher mechanical properties of tri-metal layers enable Cu-Sn-Zn to better handle the mechanical torque, dynamic stress and the duty cycle required of these components. This significantly reduces the number of component failures and costly field repairs. Based on the multitude of advantages and competitive cost, Cu-Sn-Zn can be used in a variety of commercial and consumer industries, including:
• Radio frequency (RF)
• Coaxial connectors
• Microwave components
• Electrical contact applications
• High Wear Applications
• Medical
• Automotive connectors
• Consumer electronics.
Additionally, the excellent corrosion resistance of Cu-Sn layers on brass substrates is also used in the automotive industry. Examples are connectors used for the car-trailer interface (12 volt). These parts have to perform under severe environmental attack, such as chlorides used in the winter for de-icing. In this regard, copper-tin layers are superior to commonly used coatings of nickel and pure tin.
RF connector industry and passive intermodulation distortion
Passive intermodulation distortion (PIM) was of little concern to the RF connector designer some years ago. Today however, with the ever increasing frequency demands, increasing transmitter power levels and more sensitive receivers, PIM has surfaced as a serious problem for GSM, DCS, PCS and other wireless services. PIM is unwanted signal or signals generated by the non-linear mixing of two or more frequencies in a passive device such as a connector or cable. If a circuit has non-linear characteristics, then the frequency components will be distorted and generate a higher order of harmonic frequency components. If the frequency distortion falls within the receiving band, they can effectively block a channel, when in fact, a carrier is not actually present. Ever tightening frequency boundaries will only exacerbate this problem.
As noted earlier, tri-metal layers are non-magnetic (i.e., diamagnetic) and therefore particularly suitable for high-frequency applications. Unlike nickel, tin-alloys are diamagnetic, which is a critical feature for high frequency signal transmission of connectors in the RF telecommunications field, high power wireless telecommunications applications such as antennas, base stations and satellite communications. Figure 11 shows four examples of acid copper-plated brass RF connectors overplated with 3.0 µm (120 μ-in.) of tri-metal. Figure 12 shows an RF filter module application where 3.0 µm (120 μ-in.) of tri-metal replaces silver plating.

Figure 11 - High frequency RF connectors.

Figure 12 - High frequency RF filter module.
There are three major causes of PIM in passive devices:
1. Poor contact junctions, which fail under dynamic flexing and vibration.
2. Components made with materials that exhibit hysteresis.
3. Contamination or oxidation.
The most common and serious nonlinearities occur where the current carrying area becomes microscopically separated. This can occur because of insufficient contact pressure, irregular contact pressure and oxidation causing a metal oxide junction, and contact impurities or corrosion.
Based on their intrinsic non magnetic properties, copper-tin alloys simply avoid interference with (RF) signals as compared to nickel and stainless steel, both of which can increase distortion by 50 dBs or more. Figure 13 compares the dBm distortion (increase) that is seen when using nickel versus copper-tin-zinc or silver. The distortion fIM3 is measured on a log scale and is ~10E20 higher for nickel (~log -110 to log -130). This increased distortion is enough to overlap with nearby receiving bandwidths. By overlapping these receiving bandwidths, carrier positions become blocked or viewed as present when they actually do not exist.
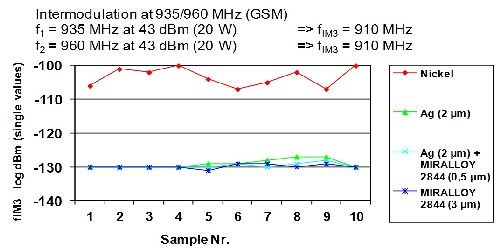
Figure 13 - Intermodulation at 935/960 MHz (GSM)
f1 = 935 MHz at 43 dBm (20 W) – 2×f1-f2 → fIM3 = 910 MHz
f2 = 960 MHz at 43 dBm (20 W) – 2×f1-f2 → fIM3 = 910 MHz
At high frequencies and as a result of the “skin effect,” where the majority of electrons are conducted at the outer surface (80 µ-in.), a stable, low porosity and highly conductive metal is critical. The improved hardness and wear resistance of Cu-Sn-Zn allows for higher compression crimping, allowing Cu-Sn-Zn to outperform silver consistently in long term contact resistance reliability. Reliability under stressful vibration and dynamic flexing applications has proven Cu-Sn-Zn’s superiority as the best performing alloy for this application. Best of all, these performance features are obtained using a standard electroplating process and at a highly competitive cost.
Decorative applications of Cu-Sn and Cu-Sn-Zn deposits
A requirement for the plating industry during the last few years has been to develop an alternative to electroplated nickel for decorative applications such as fashion jewelry, accessories and watches. These articles are typically plated with a nickel deposit to avoid diffusion of the basis material through the top layer of gold. In other cases, nickel is also often used as the final coating.
If nickel comes into intense contact with the human skin, approximately 15 to 20% of women and approximately 5% of men will show an allergic reaction to nickel. The actual illness “nickel allergy” is preceded by a so-called sensitization phase. After the first contact of the allergen nickel with the immune system of the human body, e.g., during ear piercing, the metal salts combine with the protein components of the blood to form so-called haptens. The immune system identifies them as foreign and develops a counter strategy. A sensitization takes place. After repeated contact with the allergen, the actual allergic illness breaks out in the form of reddening of the skin, small blisters or inflammation.
To avoid such exposure to potentially allergenic nickel, a number of countries have taken measures to establish consumer protection against the nickel allergy. An example is the European community, which introduced the EU directive 94/27/EG. This directive with the technical standards EN 1810, EN 1811, EN 12472, was converted into national law within all countries of the European community in January 2000. Whether there are legal requirements or not, the nickel allergy issue also affects the United States and other countries.
Summary
The deposits of single-metal processes cannot be varied to a large extent. However, the plating of alloys offers the possibility of specifically tailoring the deposit by selecting the alloy metals and the alloy composition. The plating of copper-tin-zinc alloys is a perfect example of this approach. By using tin-alloys, plating requirements such as the plating of corrosion-resistant, diamagnetic or antiallergenic deposits can be fulfilled with a single alloy process. Due to the development progress of the plating of tin-alloys that has been made within the last few years, these processes are now being used for a variety of applications. These range widely from decorative parts such as zippers and fashion jewelry to functional deposits, for example the plating of automotive parts and RF-connectors.
Fields of application for tri-metal Cu-Sn-Zn coatings offer a wealth of possible applications. Copper-tin-zinc layers do not have the allergenic effect of nickel coatings. Tri-metal copper-tin-zinc therefore is an excellent alternative to nickel. It even offers possible solutions to complicated coating tasks and can also be used in combination with other metals, e.g., copper as an undercoat, or with precious metals as final layers.
Copper-tin-alloys are diamagnetic and, therefore, are applicable as deposits for high-frequency RF connector applications. Tin-alloys show a lower passive intermodulation (PIM) at high transmission frequencies versus nickel. The use of Cu-Sn-Zn alloys on brass substrates is superior to nickel in electrical interference and to silver in corrosion. Furthermore, Cu-Sn-Zn layers offer improved wear resistance and low porosity. The thickness distribution and alloy consistency of the deposit is excellent. This enables the total thickness to be reduced whereby typically consistent 2.0-3.0 µm (80-120 μ-in.) of alloy can be easily deposited.
Copper-tin alloys white bronze processes will deposit over most substrates and maybe overplated with almost every metal. We look for this alloy and process to find expanded applications and present significant cost-saving opportunities for many electronic applications. Since white bronze processes were introduced, research has continued and other alloys of bronze may now be electroplated for special jewelry applications. Yellow bronze is an 18 karat color bronze alloy containing typically 80% copper and 20% tin. This process is intended as an undercoat to very thin gold deposits. When the gold wears through, the yellow bronze deposits will exhibit the original color, whereas if white bronze or nickel were the undercoat, the lack of gold would be obvious.
Table 3 gives an overview of the performance features of Cu-Sn-Zn compared to the single metal systems which are most commonly used. Although slightly more expensive than standard commodity metals such as nickel, copper and tin, tri-metal provides key advantages in corrosion protection, mechanical and electrical properties, non-allergenic and diffusion underplate capability. Its versatility and competitive cost will clearly find expanded roles as a high performance coating.
Table 3 - Summary of tri-metal Cu-Sn-Zn properties.
| Non-allergenic | Leveling | Brightness | Hardness | Corrosion protection | Color | Diffusion barrier | Price | Rack/Barrel plating | Subsequent plating |
Nickel | ▼ | ▲ | ▲ | ▲ | ► | ▲ | ▲ | ▲ | ▲ | ▲ |
Tin | ▲ | ► | ▲ | ▼ | ► | ▲ | ▼ | ▲ | ▲ | ▼ |
Tin/Lead | ▲ | ▼ | ► | ▼ | ► | ► | ▼ | ▲ | ► | ▲ |
Cobalt | ▼ | ► | ► | ▲ | ▲ | ▲ | ▲ | ▼ | ▲ | ▲ |
Palladium | ▲ | ▼ | ▲ | ▼ | ► | ▲ | ▲ | ▼ | ▲ | ▲ |
Zinc | ▲ | ▲ | ▲ | ▼ | ► | ► | ▼ | ▲ | ▲ | ▼ |
Acid copper | ▲ | ▲ | ▲ | ▼ | ▼ | ▼ | ▼ | ▲ | ▲ | ▲ |
White Bronze | ▲ | ▼ | ▲ | ▲ | ▲ | ▲ | ▲ | ► | ▲ | ▲ |
Yellow Bronze | ▲ | ▲ | ▲ | ► | ► | ▼ | ▲ | ► | ▲ | ▲ |
| ||||||||||
Low performance | ▼ | |||||||||
Average performance | ► | |||||||||
High Performance | ▲ | |||||||||
Additional reading
1. Klaus Leyendecker and Franz Glaser, Tin-Alloy Deposition for Decorative and Functional Applications, White Paper, Umicore Galvanotechnik, Schwäbisch Gmünd, Germany.
2. K. Leyendecker and R. Carlos da Silva Filho, Alternatives to Electroplated Nickel: Examples and Requirements from Practical Experience, White Paper, Umicore Galvanotechnik, Schwäbisch Gmünd, Germany; Umicore Brasil, Sao Paulo, Brazil.
3. D. Weinstein, Passive Intermodulation Distortion in Connectors, Cables and Cable Assemblies, White paper, Amphenol Corp., Danbury, Conn.; ieee.li/pdf/essay/passive_imd.pdf.
Related Content
Products Finishing Reveals 2023 Qualifying Top Shops
Each year PF conducts its Top Shops Benchmarking Survey, offering shops a tool to better understand their overall performance in the industry. The program also recognizes shops that meet a set of criteria to qualify as Top Shops.
Read MoreHow to Maximize Nickel Plating Performance
The advantages of boric acid-free nickel plating include allowing manufacturers who utilize nickel plating to keep up the ever-changing regulatory policies and support sustainability efforts.
Read MoreInnovation in Plating on Plastic
Plating on advanced plastics solution offers improved adhesion, temperature resistance and cost savings.
Read MorePossibilities From Electroplating 3D Printed Plastic Parts
Adding layers of nickel or copper to 3D printed polymer can impart desired properties such as electrical conductivity, EMI shielding, abrasion resistance and improved strength — approaching and even exceeding 3D printed metal, according to RePliForm.
Read MoreRead Next
A ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read More























.jpg;maxWidth=300;quality=90)







