An Improvement in the Zincate Method for Plating on Aluminum
Investigations were conducted into the sodium zincate solution used for treating aluminum prior to its being plated. Measurements of the adhesion of the nickel plate to aluminum and its alloys were done to check the effect of modifications made to this solution.
Editor’s Note: This paper was originally published as T.E. Such & A.E. Wyszynski, Plating, 52 (10), 1027-1034 (1965). A printable pdf of this paper can be accessed and printed HERE.
ABSTRACT: Investigations were conducted into the sodium zincate solution used for treating aluminum prior to its being plated. Measurements of the adhesion of the nickel plate to aluminum and its alloys were done to check the effect of modifications made to this solution. The presence of nickel in the zincate solution was found to be the most important factor in producing the results desired and enabled adherent nickel coatings to be electrodeposited from normal bright plating baths, without an intervening layer of copper or copper alloy electroplate being necessary. The properties of the immersion layer produced from this sodium zincate solution are discussed. Process sequences suitable for various types of alloy are described. Corrosion tests were conducted on aluminum plated with various nickel and chromium coatings and the results obtained are summarized.
Introduction
While countless applications of aluminum and its alloys do not require their surfaces to be treated in any way, there are many instances where their serviceability can be improved by some finishing process. Although the most popular finishes are those produced by anodizing or chromate passivating, electrodeposited coatings are important for certain applications, particularly in providing a lustrous appearance for decoration and when aluminum has to be soldered either to itself or to another metal. However, the electrodeposition of adherent metal coatings onto aluminum does present some difficulty due to the ease with which this metal forms an oxide layer on its surface. Therefore, although electrodeposited finishes on aluminum were first recorded some fifty years ago1 and have been often investigated since that time, more complex pretreatment processes are still necessary when plating onto aluminum than, for example, onto steel or brass.
The well-known zincate process is the most widely used technique for preparing aluminum for plating. The simplest form of this solution consists only of zinc oxide dissolved in sodium hydroxide. The sodium hydroxide dissolves the surface layer of oxide off the aluminum and zinc is then deposited onto this fresh surface by galvanic action. This thin layer of zinc prevents the oxide from reforming and acts as an adherent base onto which other metals - most frequently copper or brass - can be deposited. Over the course of years, the basic zincate solution has been adjusted from time to time in efforts to make it give a more adherent immersion deposit or render it more versatile in coping with a wider range of alloys, or to confer other benefits to a process which was often found to be more of an art than a science. Several metals have been mentioned in the literature as being beneficial when added to the zincate solution and of these copper3-5 and iron6 appear to be the most widely used in industrial practice. Complexing agents are needed to retain these other metals in solution - cyanide4,5,7 and tartrate6,8 being typical examples of these. Other anions such as chloride6 or nitrate9 have also been found to give improvements.
Since nickel, usually with a top-coat of chromium, is the most important metal to be electroplated on aluminum, it was considered that this metal might be advantageous if it were incorporated into the zincate dip.
The object of this work was, therefore, to formulate a zincate solution containing nickel which would be capable of producing a modified immersion deposit on aluminum, to determine the physical and chemical characteristics of this deposit, to assess the adhesion of nickel and some other commonly electroplated metals onto this immersion film both on aluminum and a wide range of its alloys, to evaluate the corrosion resistance of the plated parts and, finally, if the previous work indicated the value of doing so, to produce suitable process sequences so as to enable this modified zincate bath to be used for commercial plating.
Experimental procedure
1. Formulation of zincate solution
The approach made to solution formulation was to start with the basic zincate solution containing sodium hydroxide and zinc oxide and, after checking which compositions of this type gave the optimum results, then to modify this solution by adding nickel and other cations together with the complexing agents which would keep them in solution in a highly alkaline medium. Different heavy metals, anions and complexing agents were added in succession to the basic formulation, singly and in combination at different concentrations, and the effectiveness of the formulation was tested, using the adhesion of nickel plate to commercial purity aluminum as the criterion of suitability.
The results, which will be discussed more fully later, did show that the addition of nickel together with cyanide and other complexants to the basic sodium zincate solution enables the production of an immersion deposit onto which the majority of metals commonly used could be electroplated satisfactorily with good adhesion.
The effects of variables such as temperature, time, cleaning cycle and aluminum alloy composition and heat treatment to which the alloy was subjected were then evaluated using the standard solution composition.
2. Determination of the nature and composition of the immersion film
The physical and chemical composition of the film was studied by chemical, metallographic and electron microscopy techniques.
3. Adhesion testing
The adhesion of the immersion deposit and the electrodeposited coating on aluminum and its alloys was evaluated qualitatively and quantitatively. The qualitative tests consisted of depositing 1 mil of the metal in question on a flat aluminum plate and then flexing this plate through 180°, until the plate fractured. In the cases of low adhesion, there was usually some exfoliation of the deposit on the side of the plate which was concave on the first bending. An exfoliated deposit could usually be manually stripped by direct pull and the force required to strip it could be qualitatively judged so that the adhesion could be graded on an arbitrary scale. If the nickel deposit did not lift spontaneously, efforts were made to lift it away from the fracture by means of a pen knife or coarse file. With well-adherent deposits this lifting never extended more than 3 mm (⅛ in.) from the fracture. More massive samples were cut through by sawing and the same methods applied at the saw-cut in attempts to lift the plate. Further qualitative tests consisted in baking a plated specimen at 315°C (600°F) for 10 minutes followed by rapid quenching in cold water and examining it for blisters.
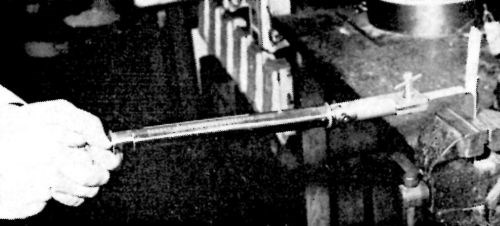
Figure 1 - Method of adhesion testing.
The quantitative determinations, which followed the pattern mapped out by the qualitative tests, were made using the method developed by Wittrock and Swanson10. The apparatus and the test method are illustrated in Fig. 1. A vise grip on a flexible coupling is attached to the spring of a spring balance 45 kg (100 lb.) capacity, calibrated in units of 0.45 kg (1 lb.). The test piece consisted of an aluminum strip or a rod rigid enough to withstand a bending force of the order of 100 lb. The strip was cleaned in a normal manner and then immersed in the pretreatment solution to a depth sufficient to leave about 2.5 cm (1 in.) at the top of the strip exposed above the solution level. The whole was then electroplated with ductile nickel, copper etc., to a thickness of about 10 mil. The deposit adhered to the surface which was treated in the solution and was non-adherent to the untreated surface from which it was detached to serve as the starting point for the test. The edges of the strip were chamfered, and the strip placed vertically in a vise. The electroformed tongue of nickel was clamped in the vise grip of the spring balance and pulled steadily at right angles to the plane of the strip. The load at which the deposit becomes detached from the basis metal is recorded as the adhesion or peel-strength expressed in lb./linear inch.
4. Trials of pretreatment baths to be used prior to zincate Immersion
Many cleaning solutions have already been found satisfactory for use with zincate immersion processes, but it was necessary to vary these in order to obtain the best adhesion of electroplate onto the very wide range of alloys it was desired to treat with this new zincate solution.
Also since we wished to plate bright nickel directly onto aluminum, the surface treatment of that metal prior to zinc immersion coating had to be adjusted so as to prevent undesirable etching of the polished surface, which would impair its ultimate appearance.
5. Evaluation of corrosion resistance
The procedures adopted to test plated EC aluminum panels were as follows:
a) Accelerated corrosion tests
- Acetic acid salt spray test according to ASTM B287-62
- Corrodkote test according to ASTM B380-61T
- CASS test according to ASTM B368-62T.
b) Outdoor exposure tests
- Static exposure on a roof near the center of Birmingham, England.
- Mobile service tests on the front of cars operating mainly in the Birmingham district.
Results and discussion
1. Effect of varying the composition and operating conditions of the zincate solution
a) Composition of the zincate solution
The presence of nickel in the zincate solution was found to be of great benefit in promoting good adhesion of nickel, plated directly onto the zinc alloy layer given by the new solution. The nickel was found in many cases to make such a difference that whereas previously the adhesion of the nickel plate to a deposit from a simple zincate bath was so low that the coating was more like an envelope, the addition of nickel to this zincate solution enabled a very adherent nickel plate to be deposited onto the same aluminum alloy. It was also found that bright nickel could be directly deposited onto this modified zinc film from conventional baths at normal acidities, thus avoiding the necessity of using dull nickel baths having a high pH.
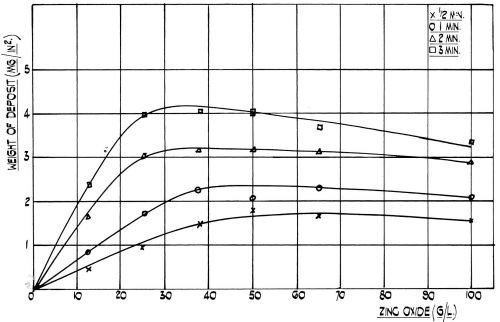
Figure 2 - Effect of zinc oxide concentration on the rate of film formation at room temperature on 99.5 per cent aluminum. (NaOH:ZnO Ratio = 5:1).
Although this beneficial effect of nickel was evident in a wide range of simple zincate solutions of various concentrations (see Fig. 2), it was considered that a dilute bath would be best, since having a low viscosity it would be more readily rinsed off than more concentrated solutions and would also penetrate more quickly into small blind holes, etc. (Note from Fig. 2 the slight decrease in coating weight given in the more concentrated and viscous solutions, which results from localized solution depletion). Therefore, a basic zincate solution containing sodium hydroxide and zinc oxide was chosen. To this basic solution nickel was added and the concentration range of this metal over which it exhibits its marked beneficial effect on adhesion was determined. The additional presence of copper was found to be of benefit when dealing with certain alloys and this metal was therefore also included in the standard formulation. These metals must not only be present in a fixed range of individual concentrations, but the ratio between zinc, nickel and copper kept within certain limits. To keep these metals in solution, the addition of complexing agents was imperative. One of these was cyanide and not only is this anion important as a complexant, but its concentration is rather critical in affecting the adhesion of the electroplate. Bengston has reported11 that, the presence of sulfates in the zincate dip or the use of zinc sulfate instead of the oxide was helpful. This was found to be the case with our solution and so sulfate was used in the formulation, although good adhesion was still obtained if the sulfate ion was partially or totally substituted by chloride or nitrate.
The final formulation of this modified zincate bath has been in commercial use as the "Bondal" bath for the last three years. This bath is tolerant to changes in composition and will produce 2m2/L (80 ft2/gal) of satisfactorily treated surface-area before the adhesion begins to deteriorate, but its life can be prolonged by maintaining the bath at the correct composition. Addition powders have been formulated containing the required ingredients in the correct ratio with respect to the quantities of zinc and caustic soda found by analysis to be necessary to replenish the bath. Therefore from the results of two simple analyses, this zincate bath can be maintained in the correct balance.
b) Effect of operating conditions
The temperature of the zincate bath and the time of immersion are chiefly responsible for the film thickness and structure. The film thickness and structure have been quite thoroughly explored before by a number of investigators,8,12,13 mainly in an attempt to find a correlation between the film weight and adhesion.
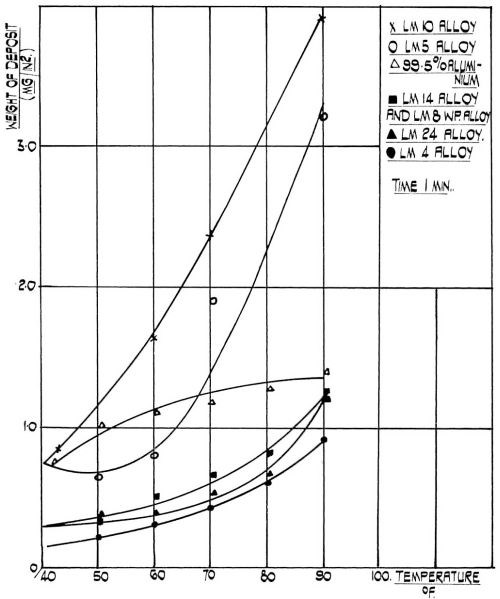
Figure 3 - Effect of temperature on the rate of film formation.
In our studies we have found that the temperature has a profound effect on the film weight of the immersion deposit on aluminum. Generally speaking, the film weight increases with temperature although its behavior is rather complex and depends on the alloy type. Figure 3 illustrates the relationship between temperature and the film weight for a range of commercial alloys. It will be noted that commercial purity aluminum takes a position intermediate between two series of alloys, i.e., alloys with high magnesium content and the alloys with high content of silicon, copper or both. It has been reported by various authors8,13-15 that the film thickness depends on the alloy composition, which in turn determines its electropotential with respect to zinc. The alloys containing magnesium are reported to be most base with respect to zinc, the commercial purity aluminum intermediate, and alloys containing copper most noble with respect to zinc. It will be seen from Fig. 3 that this relationship is most pronounced at higher temperature. It can also be seen that the behavior of commercial purity aluminum is somewhat different from the behavior of its alloys inasmuch as the temperature coefficient decreases with temperature for commercial purity aluminum, whereas for aluminum alloys it increases with temperature. The minimum on a curve for alloys containing 3-6 per cent Mg might be of electrochemical importance, but its significance cannot be visualized in the context of this work.
The rate of film formation can be controlled by the temperature of the zincate solution. It can be seen from Fig. 3 that the rate of film formation is between four and six times faster at 32°C (90°F) than it is at room temperature. Although the same film thickness can be obtained by increasing the time of treatment, the actual rate of film formation cannot be increased. We found that the rate of film formation is at least as important as the actual film thickness and that by varying the rate of film formation, good adhesion can be obtained even on alloys on which the adhesion was poor before. At a given temperature and pre-cleaning cycle, the film thickness obtained on aluminum and its alloys is proportional to the time of immersion. The rate of film formation, fast at first, progressively decreases and virtually reaches saturation. This is to be expected from a displacement reaction which is not catalyzed, for it relies on the galvanic effect which should cease after a continuous unbroken layer of the more noble metal was deposited on the more base one. Therefore, the significance of time after the first two minutes has probably been over-emphasized.
At a constant temperature and immersion time, the pre-treatment cycle affects the rate of film formation. It was found, for instance, that the rate of film formation on aluminum which was soak cleaned in a warm alkaline cleaner is slower than on aluminum which was cathodically cleaned in a cold cleaner. The frequently used technique of double zincating, that is dissolution of the zinc film first formed by immersion of the aluminum in nitric acid and then reformation of the zinc layer by dipping the aluminum in either the same or a different zincate solution, was tried with our modified zincate solution. It was found to give a definite improvement in adhesion. Previous workers' results, which showed that the rate of film formation on aluminum which has been previously treated in a zincate solution is slower than it otherwise would be on a fresh aluminum surface, have been con-firmed with this modified zincate solution.
2. Properties of the immersion film
Chemical analysis of a typical film showed that it contained 86 percent zinc, 8 percent copper and 6 percent nickel. The film is most probably an alloy rather than a chemical compound, but no data can be quoted to substantiate this assumption. The thickness of the film, which is only about 0.01 mil (0.25 microns), is not great enough to permit the analysis or definite identification of the phase present.
A preliminary study with an electron microscope showed that the film was too amorphous and contained too much included alumina to get conclusive results by this method. The diffraction patterns also did not reveal anything conclusive.
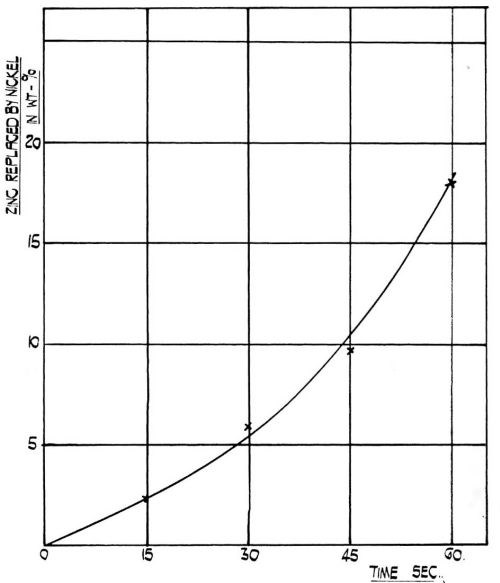
Figure 4 - The rate of replacement of zinc by nickel at room temperature in the film obtained on 99.5 percent aluminum from modified zincate solution.
One particular property of this modified zincate film is the readiness with which it reduces nickel from a sulfate solution and incorporates it in the film. It was established that the film is quite inert in a solution containing 300 g/L sodium sulfate, 30 g/L sodium chloride and 40 g/L boric acid at pH value of 4.2. When, however, the sodium sulfate is replaced quantitatively by crystalline nickel sulfate there is a rapid reaction and nickel is incorporated in the film. The rate of incorporation is illustrated in Fig. 4. It is a significant fact that the adhesion of the subsequent nickel deposit decreases with the amount of nickel incorporated after reaching a maximum at approximately 30 sec immersion. Although this has yet to be confirmed, it is possible that the adhesion mechanism is connected with the incorporation of nickel into the immersion deposit and that during the first few seconds of deposition, a reduction of nickel occurs inside the immersion zincate film, and the subsequent electrodeposition of nickel occurs on the nickel film deposited by reduction - not necessarily only on the surface of the modified zinc coating but also inside it.
A control experiment showed that an immersion zinc deposit obtained from a conventional zincate solution did not show any incorporation of nickel after 60 sec immersion in a nickel solution of the composition given above.
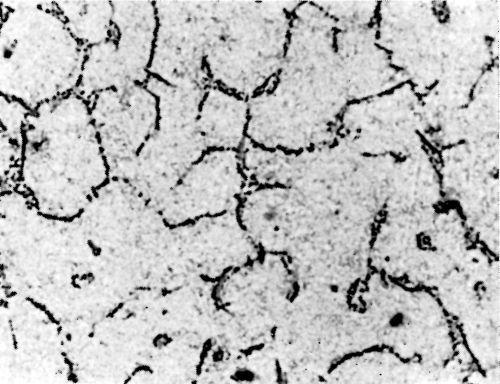
Figure 5 - Surface of LM8M alloy after polishing.
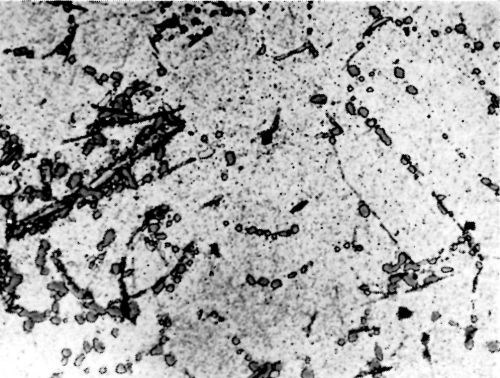
Figure 6 - Surface of LM8W alloy after polishing and etching in 0.5 percent HF solution.
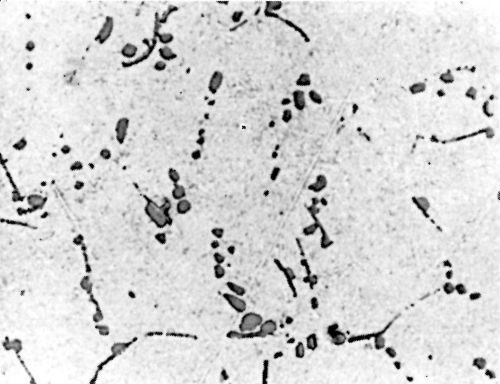
Figure 7 - Surface of LM8WP alloy after polishing.
Metallurgical investigation of the deposition mechanism showed that the surface condition is not the only factor which will affect the adhesion. The precipitation and aging treatment to which aluminum alloy is subjected will also have a decisive effect. For example, although it was impossible to detect any significant difference in the microstructures of LM8 alloy* after solution treatment at 520°C (970°F) and after solution and precipitation treatment, the adhesion to solution-treated LM8 alloy was excellent (100 lb./in.), whereas the adhesion to the same alloy when it was both solution and precipitation treated was poor (26 lb./in.). On the other hand, although there was a considerable difference in the microstructure between LM8 alloy in the as-cast condition and after solution heat treatment, the adhesion in both cases was comparable (80 and 100 lb./in. respectively). Figures 5, 6 and 7 show the microstructure of the alloy. It will be seen that, in spite of the similarity of structure in Figs. 6 and 7, the adhesion is different, and despite the difference of structure in Figs. 5 and 6, the adhesion is similar. This is most probably due to the coherent precipitate, which would be formed during the aging process and yet would be invisible under an optical microscope, affecting the surface condition of the alloy and so giving galvanic differences over the surface. Presumably after solution heat treatment, the intermetallics are so uniformly dispersed as to produce a consistent potential difference over the surface of the alloy when this is immersed in the zincate solution. The significant difference between the appearance of the metal surface after stripping the nickel deposit from LM8M, LM8W and LM8WP was that, in the case of the first two alloys, the separation occurred in the basis metal all along the line of peel, whereas in the latter case there were only relatively few deep tears in the basis metal, although the photomicrographs revealed that the aluminum adhered to the nickel, after the nickel deposit was separated mechanically from the aluminum base. This is illustrated in Figs. 8(a) and (b), which shows the aluminum basis metal and the nickel strip detached. It will be seen from the photographs that the structures are virtually mirror images of each other and that although the adhesion is low (26 lb./in.) the separation occurs in the basis metal. It is possible that the treatment of the LM8 alloy in the modified zincate solution results in the partial disintegration of the surface, producing a layer whose tensile strength is lower than the adhesion of the nickel deposit to this layer.
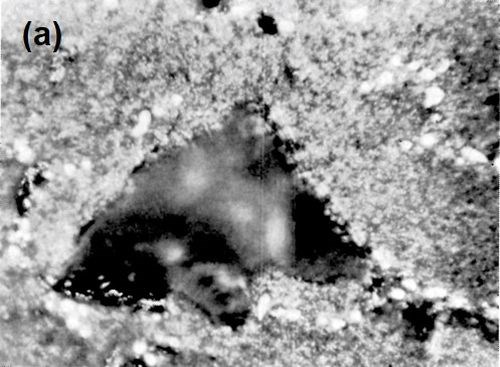
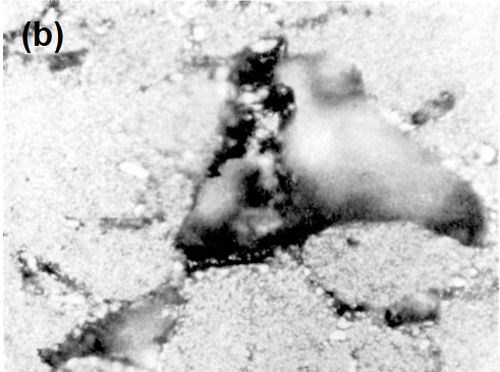
Figure 8 - (a) Surface of LM8WP alloy after removal of electrodeposited nickel by peeling; (b) nickel foil removed from LM8WP alloy by peeling.
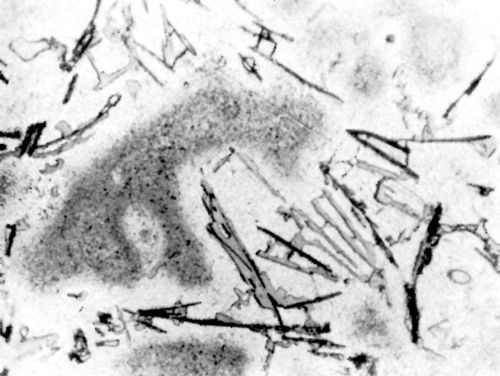
Figure 9 - Surface of LM4 alloy after polishing and dipping in modified zincate solution.
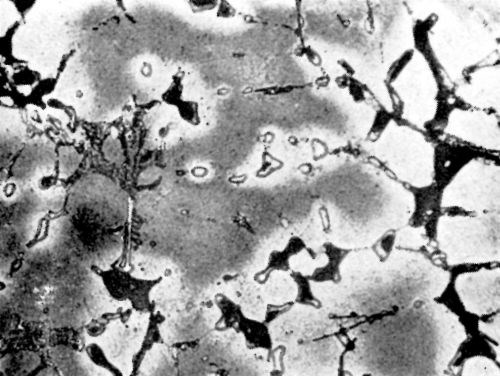
Figure 10 - Surface of LM12WP alloy after polishing and dipping in modified zincate solution.
In order to ascertain if there is any relationship between the adhesion of nickel to different alloys and the appearance of the alloy surface after it was treated in the modified zincate solution, photomicrographs were prepared of alloy surfaces after treatment. Figures 9 and 10 show the typical pattern obtained on LM4 and LM12WP.** Although the appearance could not be correlated to the adhesion obtained, Figs. 9 and 10 show the film pattern caused most probably by the thickness of the zincate film varying on different phases of the alloy.
The evidence for this is based on an investigation conducted on an electron beam microanalyzer, but so far confined to one alloy - LM8M. The microstructure of this alloy is shown in Fig. 5. Both the acicular and spherical particles have been found to be α-(Fe, Mn, Si, Al) and these are contained in a fine structure of Al-Si eutectic, with some dendrites of aluminum also present.
The surface of a specimen of this alloy was polished to a metallographic standard and treated with the modified zincate solution using the "double dip" technique. Examination with the micro-analyzer was then made of the coated surface.
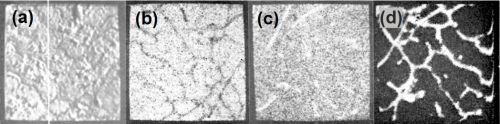
Figure 11 - Electron beam microanalysis of aluminum alloy LM8M after dipping in modified zincate solution: (a. electron image; (b. zinc x-ray image; (c. iron x-ray image; (d. silicon x-ray image.
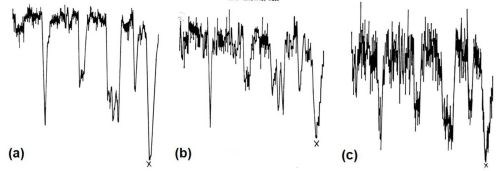
Figure 12 - Disposition of elements in modified zincate film on LM8M alloy: (a) zinc x-ray trace; (b) copper x-ray trace; (c) nickel x-ray trace.
Figure 11(a) shows the general electron image found by backscatter of electrons from the region chosen. Figure 11(b) shows the surface scanned with respect to zinc x-ray emission. Little variation in thickness of film is apparent over the matrix, i.e., interior of dendrites, but dark patches at the boundaries show that little or no zinc is present in these regions. Figures 11(c) and (d) show the field of 11(b) scanned to detect iron and silicon emissions, respectively, and indicate that thinning of the modified zincate film correlates with the disposition of both silicon and α-(Fe, Mn, Si, Al) particles. Figure 12 shows pen recorder traces, obtained by slow scanning along the line indicated in Figure 11(a), by a technique in which the electron beam penetrates through the modified zincate film. In this way a semi-quantitative estimate of elements present in the volume penetrated by the beam can be obtained. The first trace, Fig. 12(a), was made with the detector sensitive to zinc emission only, while Figs. 12(b) and (c) give traces corresponding to the concentration of the minor elements copper and nickel. Concentration of each element varies in a similar way indicating that composition of the film remains constant over the surface. Variations in intensity of emission over the traverse indicate differences in modified zincate film thickness. Further traces with the detector arranged to be sensitive to iron and silicon, Figs. 13(a) and (b), respectively, confirm that the regions rich in iron and silicon coincide with a decrease in film thickness. The thickness remains fairly constant at about 0.02 mil (0.5 microns) over the dendrites but is thinner than 0.004 mil (0.1 microns) - possibly disappearing entirely - in regions where iron- and silicon-bearing constituents are present.
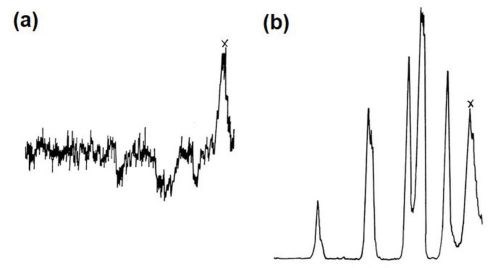
Figure 13 - Disposition of alloying elements in LM8M alloy: (a) iron x-ray trace; (b) silicon x-ray trace.
3. Adhesion of plated coatings
The use of the process sequences described below enables adherent coatings of both dull and bright nickel to be plated from conventional Watts baths at normal acidities (pH range 3 to 5) onto commercial purity aluminum and its alloys selected from the types most frequently employed in the industrial field. The fracture or sawing and the heating plus quenching tests were used on all alloys to check the adhesion obtained. The quantitative peel test was used on some samples to obtain more detailed information to correlate with these qualitative results (See Table 1). It was found that alloys of the following series could be satisfactorily plated using the modified zincate dip - 1000, 2000, 3000, 5000 and 6000. Typical wrought alloys to which nickel had good adhesion were 1100 and purer aluminum up to 99.99 percent, 2014, 2017, 3003, 5050, 5052, 5056, 5154, 6053, 6061, 6063 and 6151. Of the casting alloys, those similar to 13, 122, 132, 142, 214, 220, 319, 356 and 380 have all been successfully nickel plated. Summing up, it seems that nickel - whether dull or bright - can be plated adherently onto any aluminum alloy containing not more than 5 percent copper, 9 percent magnesium, 1.5 percent manganese or 13 percent silicon, once these have been treated in the "Bondal" dip. It must be emphasized that this result can be obtained without any baking treatment, such as is vital for some processes used for plating aluminum.
4. Pretreatment before modified zincate dip
As stated above, the modified zincate dip is most versatile and so can be used with satisfactory results on many types of aluminum alloy. For commercial purity aluminum and alloys containing a total of less than 1.5 percent of alloying elements, the simple process sequence given below will give excellent adhesion of nickel and many other electroplated coatings:
- Trichoroethylene degrease or soak clean in a hot non-silicated, non-etch cleaner.
- Water rinse.
- Cathodically clean in a non-silicated caustic-based cleaner.
- Water rinse.
- Nitric acid dip (50 percent by volume).
- Water rinse.
- Immerse in "Bondal" modified zincate dip for 1 to 2 minutes at 16-30°C (60-85°F).
- Water rinse.
- Electroplate with desired metal using conventional dull or bright plating baths.
For casting alloys containing large amounts of silicon or copper, such as 13, 380 or 122, it is best to use a double immersion in the modified zincate dip. Alloys containing more than 3 percent magnesium, for example 220 or 5056, should be etched in hot, dilute sulfuric acid instead of the nitric acid dip in stage 5 of the above sequence.
Many metals other than nickel can be deposited even on the more complex aluminum alloys using a suitable sequence. For example, hard chromium can be deposited directly onto 13 or 380 alloys from baths not containing silicofluoride, provided a mixture of nitric and hydrofluoric acids is used in stage 5 of the standard process sequence. Copper and brass can also be deposited directly onto aluminum using cyanide baths as can zinc, cadmium, and silver. Tin is best plated from a stannous sulfate solution rather than a sodium stannate bath. If a certain bath cannot be used for direct plating as in the case of acid copper or high efficiency chromium, or where an under-coat is desirable, such as with gold, then the desired metal can be deposited over an under-coat of nickel.
5. Corrosion tests on plated aluminum
The accelerated tests showed that the corrosion resistance of aluminum plated with 1.2 mils of nickel depended, just as with other metals, on the nature of the nickel and the topcoat of chromium. If bright nickel plus 0.01 mil of chromium were used, the samples failed badly in the tests, while the use of duplex nickel or micro-cracked chromium top-coats resulted in a much better performance and plated aluminum then behaved similarly to steel parts plated with the same type of coating, both the steel and aluminum panels failing after almost exactly the same period of exposure to the accelerated tests. For example, aluminum and steel specimens plated with 0.03 mil of micro-cracked chromium over 1.2 mil of bright nickel still had ASTM ratings of 9 after either two cycles of the Corrodkote test or 240 hours in Acetic Acid Salt Spray. Samples plated with 1.2 mil of duplex nickel plus 0.01 mil of regular chromium had ratings of 9 after two Corrodkote tests or one CASS test. Crack-free chromium gave a variable performance; sometimes it was very good, but if a few pores were present in this coating, then these would quickly result in penetration of the underlying nickel and attack on the aluminum. During the course of these tests, it was observed that aluminum parts plated after treatment with the modified zincate solution exhibited much less lateral corrosion at the aluminum/electroplate interface than did those plated with copper, nickel and chromium after immersion in a simple zincate solution.
The outdoor exposure tests confirmed the accelerated tests, with duplex nickel and micro-cracked chromium again showing their benefits, but heavy deposits of crack-free chromium proving to be worse than the usual chromium layer of 0.01 mil.
Although extensive tests were carried out, they can be summarized by stating that in all cases, except crack-free chromium, the benefits of improved nickel and chromium coatings previously found most suitable for steel and zinc alloy were found applicable to aluminum. It has become increasingly obvious as more corrosion tests have been performed and the behavior of various commercially plated articles observed in service, that it is the type of plated nickel/chromium coating that is the most important factor. The life of this is obviously the same on aluminum as, for example, on zinc alloy.
Conclusions
The presence of nickel ions in the sodium zincate solution used for immersion treating aluminum has been found beneficial. It has enabled nickel, including bright nickel, and other metals to be directly and adherently plated onto aluminum and a wide range of its alloys, without any plated undercoat of copper or copper alloy being necessary. Nor is baking required. The corrosion resistance of aluminum, nickel and chromium plated by this process depends on the nature of the coating, and duplex nickel or micro-cracked chromium is therefore recommended for articles which are to be used in severely corrosive conditions. Bright nickel plus regular chromium is, however, quite satisfactory for normal indoor service. That this is so is confirmed by the variety of articles which have been successfully commercially plated by this proprietary process, which include domestic hollow-ware, cosmetic containers, buttons and badges, and components of electric fires and cookers, cameras, dispensing machines and general engineering equipment, while its use on parts for outdoor service is typified by automobile and motor-cycle trim.
Acknowledgements
The authors are indebted to the directors of W. Canning & Co. Ltd., for permission to publish this paper. They wish to thank their colleagues, particularly Miss J.E. Parker and Mr. G.R. Davies, for their assistance in the experimental work. In addition, they are most appreciative of the work done by Dr. J.K. Dennis, also of the Canning Research Laboratories, who produced the optical photomicrographs, and for the assistance rendered by the Imperial Aluminum Co. Ltd. who performed the electron beam analysis.
References
1. Q. Marino, Brass World, 9, 29 (1913).
2. S. Wernick & R. Pinner, The Surface Treatment and Finishing of Aluminum, Teddington, Robert Draper, 1964; Chapters 13-16.
3. J. Korpiun, U.S. Patent 2,142,564 (1939).
4. F. Passal, U.S. Patent 2,662,054 (1953).
5. J. Patrie, U.S. Patent 2,745,799 (1956).
6. W. Zelley, U.S. Patent 2,676,916 (1954).
7. J. Korpiun, U.S. Patent 2,418,265 (1947).
8. W. Bullough & G.E. Gardam, J. Electrodep. Tech. Soc., 22, 169 (1947).
9. W. Zelley, J. Electrochem. Soc., 100, 328 (1953).
10. H.J. Wittrock & L. Swanson, Plating, 49, 880 (1962).
11. H. Bengston, Trans. Electrochem. Soc., 88, 307 (1945).
12. G.L.J. Bailey, J. Electrodep. Tech. Soc., 27, 233 (1951).
13. F. Keller & W.G. Zelley, J. Electrochem. Soc., 97, 143 (1950).
14. H. Richaud, Revue De Aluminum, 881, July 1961.
15. V.I. Lainer & Yu A. Velichko, Vestnik Mashinostroenya, 37, 48 (1957).
About the authors (written in 1965)

T.E. Such studied chemistry at the University of Birmingham, England, from which he obtained his B.Sc. in 1945. He then spent six years at Needle Industries Limited, first as a chemist and from 1948 as chief chemist. In 1951, he joined the automobile accessory firm of Wilmot-Breeden Limited, as senior electrochemist. He then became deputy technical manager and later technical manager, at Ionic Plating Company Limited. Since 1958, Mr. Such has been head of the research laboratory of W. Canning and Company, Limited. Mr. Such was elected a Fellow of the Royal Institute of Chemistry in 1963. In addition to the American Electroplaters' Society, he is a member of the Institute of Metal Finishing, the Society of Chemical Industry, and the British Joint Corrosion Group.

A.E. Wyszynski obtained his technical education at Leicester and at Birmingham College of Advanced Technology, England, after his war-time service with the Polish Army in the Middle East. He was employed at Metalastik Limited, Leicester, as a research chemist, investigating rubber to metal bonding and the effect of electrodeposited brass structure and composition on the properties of the bond. Mr. Wyszynski is how a research chemist at W. Canning and Company, Limited, Birmingham, where he is investigating the properties and solutions used for bright plating, as well as the properties of the deposits obtained from them.
Related Content
Innovation in Plating on Plastic
Plating on advanced plastics solution offers improved adhesion, temperature resistance and cost savings.
Read MoreSuccessful South African Plater Beating the Odds
Remaining focused on quality and reliability, Team Plating Works stays profitable in a volatile and challenging economy.
Read MoreHow to Maximize Nickel Plating Performance
The advantages of boric acid-free nickel plating include allowing manufacturers who utilize nickel plating to keep up the ever-changing regulatory policies and support sustainability efforts.
Read MoreNanotechnology Start-up Develops Gold Plating Replacement
Ag-Nano System LLC introduces a new method of electroplating based on golden silver nanoparticles aimed at replacing gold plating used in electrical circuits.
Read MoreRead Next
Education Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read More





















