Electro-codeposition of MCrAlY Coatings for Advanced Gas Turbine Applications - 2nd Quarterly Research Report
In this quarter, focus was on planned research on the effect of current density and powder density on CrAlY(Ta) particle incorporation in electro-codeposited Ni-CrAlY(Ta) composite coatings.
Editor’s Note: This NASF-AESF Foundation research project report covers the second quarter of project work (April-June 2018) on an AESF Foundation Research project at the Tennessee Technological University, Cookeville, Tennessee. A printable PDF version of this report is available by clicking HERE.
Summary: In this quarter, we focused on the proposed research activities in Task 1, i.e., the effect of current density and powder density on CrAlY(Ta) particle incorporation in electro-codeposited Ni-CrAlY(Ta) composite coatings. Ni-based alloy specimens were plated at current densities ranging from 5 to 20 mA/cm2 in a rotating barrel containing ball-milled CrAlY and CrAlYTa powders. The particle incorporation increased from 30-33 vol% to ~40 vol% when the current density was decreased from 20 to 5 mA/cm2. The particle density also slightly affected the particle incorporation. The heavier CrAlYTa powder (5.5 g/cm3) exhibited higher particle incorporation than the CrAlY powder without Ta (4.5 g/cm3).
Technical report
I. Introduction
To improve high-temperature oxidation and corrosion resistance of critical superalloy components in gas turbine engines, metallic coatings such as diffusion aluminides or MCrAlY overlays (where M = Ni, Co or Ni+Co) have been employed, which form a protective oxide scale during service.1 The state-of-the-art techniques for depositing MCrAlY coatings include electron beam-physical vapor deposition (EB-PVD) and thermal spray processes.1 Despite the flexibility they permit, these techniques remain line-of-sight which can be a real drawback for depositing coatings on complex-shaped components. Further, high costs are involved with the EB-PVD process.2 Several alternative methods of making MCrAlY coatings have been reported in the literature, among which electro-codeposition appears to be a more promising coating process.
Electrolytic codeposition (also called “composite electroplating”) is a process in which fine powders dispersed in an electroplating solution are codeposited with the metal onto the cathode (specimen) to form a multiphase composite coating.3,4 The process for fabrication of MCrAlY coatings involves two steps. In the first step, pre-alloyed particles containing elements such as chromium, aluminum and yttrium are codeposited with the metal matrix of nickel, cobalt or (Ni,Co) to form a (Ni,Co)-CrAlY composite coating. In the second step, a diffusion heat treatment is applied to convert the composite coating to the desired MCrAlY coating microstructure with multiple phases of β-NiAl, γ-Ni, etc.5
Compared to conventional electroplating, electro-codeposition is a more complicated process because of the particle involvement in metal deposition. It is generally believed that five consecutive steps are engaged:3,4 (i) formation of charged particles due to ions and surfactants adsorbed on particle surface, (ii) physical transport of particles through a convection layer, (iii) diffusion through a hydrodynamic boundary layer, (iv) migration through an electrical double layer and finally, (v) adsorption at the cathode where the particles are entrapped within the metal deposit. The quality of the electro-codeposited coatings depends upon many interrelated parameters, including the type of electrolyte, current density, pH, concentration of particles in the plating solution (particle loading), particle characteristics (composition, surface charge, shape, size), hydrodynamics inside the electroplating cell, cathode (specimen) position, and post-deposition heat treatment if necessary.3-6
There are several factors that can significantly affect the oxidation and corrosion performance of the electrodeposited MCrAlY coatings, including: (i) the volume percentage of the CrAlY powder in the as-deposited composite coating, (ii) the CrAlY particle size/distribution, and (iii) the sulfur level introduced into the coating from the electroplating solution. This three-year project aims to optimize the electro-codeposition process for improved oxidation/corrosion performance of the MCrAlY coatings. The three main tasks are as follows:
- Task 1 (Year 1): Effects of current density and particle loading on CrAlY particle incorporation.
- Task 2 (Year 2): Effect of CrAlY particle size on CrAlY particle incorporation.
- Task 3 (Year 3): Effect of electroplating solution on the coating sulfur level
In this reporting period, we focused on the proposed research activities in Task 1, particularly the study of the effect of current density on CrAlY particle incorporation in the electro-codeposited coatings.
II. Experimental procedure
2.1. Substrate alloys and powders
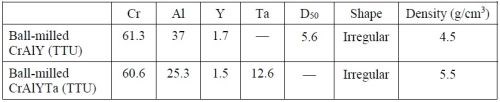
Substrates were made from available nickel-based alloys including Ni 200 (>99.0 Ni, with 0.25 Cu-0.40 Fe-0.35 Mn-0.15 C-0.35 Si-0.01 S max., in wt%) and René 80 (Ni-3.0 Al-14.1 Cr-9.7 Co-4.3 W-4.0 Mo-5.0 Ti-0.18 C in wt%, 130B-200 Zr-7 S in ppmw). Disc specimens (1.6 mm thick, ~17 mm in diameter) were cut with an abrasive cutting saw. The specimens were ground to #600 grit using SiC grinding papers, followed by grit blasting with #220 Al2O3 grit, and were then ultrasonically cleaned in hot water and acetone. Pre-alloyed CrAlY and CrAlYTa powders (Table 1) were utilized in the electro-codeposition experiments. The powders were fabricated at TTU and the detailed powder making process was described in the 2018-Q1 Report.
Table 1 - Compositions (wt%) and properties of the powders used in electro-codeposition.
2.2. Electro-codeposition experiments
A rotating barrel system shown in Fig. 1 was employed in the electro-codeposition experiments; details can be found in the 2018-Q1 Report. Watts nickel plating solution was used and the nickel anode was placed outside of the barrel along with a mechanical stirrer and heating coil. The specimens were plated at 50°C with a pH level of 3.7-3.9. The CrAlY(Ta) particle concentration in the plating bath was kept at 20 g/L and the barrel rotation speed at 7 RPM. To understand the effect of current density on particle incorporation, specimens were plated at different current densities, ranging from 5 to 20 mA/cm2.
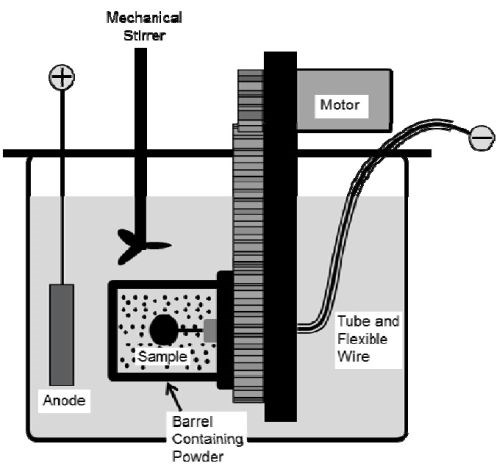
Figure 1 - Schematic of the barrel system.
2.3. Coating Characterization
The Ni-CrAlY(Ta) composite coatings were characterized using scanning electron microscopy (SEM) equipped with energy dispersive spectroscopy. Prior to metallographic sample preparation, the specimens were copper-plated to improve the edge retention. To determine the volume fraction of the incorporated CrAlY particles, multiple backscattered electron images were taken from different locations along the coating cross-section, which were then processed using the ImageJ software. The brightness and contrast of the image were adjusted by setting a proper threshold such that the particles were separated from the background. The area fraction of the CrAlY particles was determined, which was assumed equivalent to its volume fraction.
III. Results and Discussion
Figure 2 shows the SEM backscattered-electron cross-sectional images of the as-deposited coatings plated at different current densities using the ball-milled powders CrAlY and CrAlYTa. Tantalum is added in MCrAlY coatings to counteract the detrimental effect of titanium from the superalloy substrate on coating oxidation resistance.7 The irregular shape of the ball-milled particles can be clearly seen in Fig. 2. It is worth noting that the coatings were plated for different time durations to obtain a similar coating thickness. It should also be pointed out that in metallographic sample preparation, if the cutting speed was too fast, separation between the coating and the substrate could occur, as what is observed in Figs. 2(c and d).
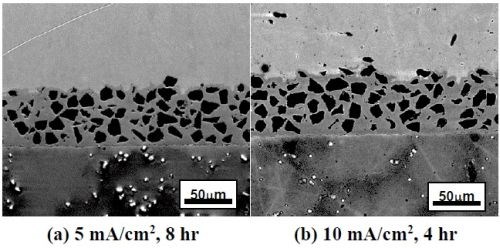
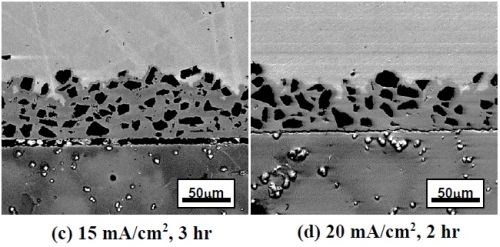
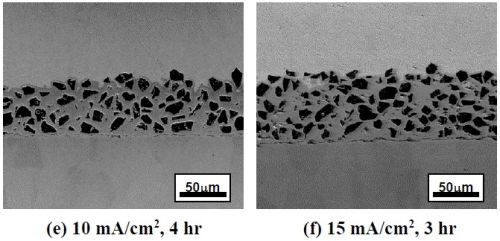
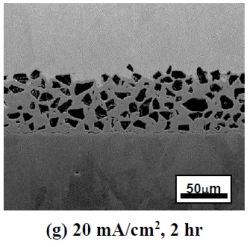
Figure 2 - SEM cross-sectional images of the coatings fabricated with two ball-milled powders at different current densities: (a)-(d) CrAlY powder; (e)-(g) CrAlYTa powder.
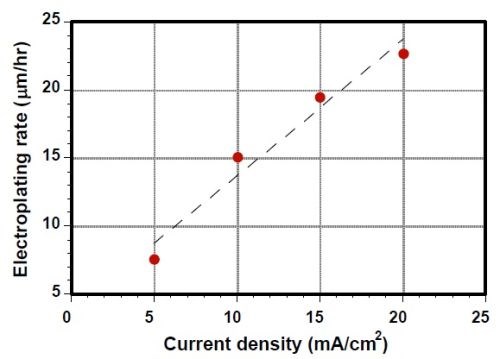
Figure 3 -Electro-codeposition rate as a function of current density.
Current density is a critical process parameter in conventional electroplating that governs the coating deposition rate. Figure 3 is a plot showing the plating rate as a function of current density for the electro-codeposited Ni-CrAlY coatings. A nearly linear relationship was observed, which was similar to that for traditional electroplating without the powders.
The effect of current density on particle incorporation depends strongly on the nature of particles and the metal deposit. Different types of relationships have been reported. The particle content in the composite coatings either increases or decreases continuously with the current density, or exhibits one or multiple peaks as a function of current density.8 Figure 4
|
|
|
|
shows the incorporation of CrAlY and CrAlYTa particles in the as-deposited Ni-CrAlY(Ta) coatings as a function of the current density. The particle incorporation increased from 30-33 vol% to ~40 vol% when the current density was decreased from 20 to 5 mA/cm2. A similar trend was reported by Bazzard and Boden for codeposition of chromium particles in a nickel matrix in the current density range of 10-40 mA/cm2.9 The particle density seemed to affect the particle incorporation slightly; the heavier CrAlYTa powder (5.5 g/cm3) led to higher particle incorporation than the CrAlY powder without Ta (4.5 g/cm3).
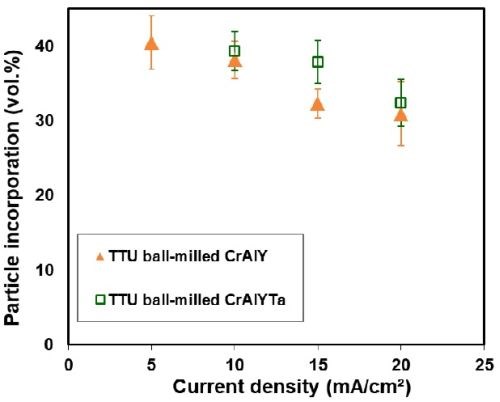
Figure 4 - Particle incorporation of ball-milled powders as a function of plating current density.
Typical MCrAlY coatings contain 8–12% Al, 18–22% Cr and up to 0.5% Y (in wt%). To form a NiCrAlY coating with 10 wt% Al, based on the chemical composition and the density of the CrAlY powder used in this study, approximately 40 vol% of CrAlY particles are needed in the as-deposited composite coatings. According to the data presented in Fig. 4, current densities in the range of 5-10 mA/cm2 are needed in order to reach the 40 vol% particle incorporation, particularly for the CrAlY powder without tantalum. For the same coating thickness, a longer plating time is needed for lower current densities. For example, to form an MCrAlY coating of ~100 μm thick, a plating time of 6.6-13.2 hr is required for such low current density levels. A realistic current density level needs to be identified for a balance of coating deposition rate and particle incorporation. An understanding of the codeposition behavior of the gas-atomized CrAlY powder with spherical shape is also needed.
IV. Future work
In the next quarter, higher current densities in the range of 40-60 mA/cm2 will be employed in the electro-codeposition process to complete the study of the effect of current density on coating particle incorporation. In addition, the influence of CrAlY particle shape on particle incorporation will be investigated.
References
1. G.W. Goward, Surf. Coat. Technol., 108-109, 73-79 (1998).
2. A. Feuerstein, et al., J. Therm. Spray Technol., 17 (2), 199-213 (2008).
3. C.T.J. Low, R.G.A. Wills and F.C. Walsh, Surf. Coat. Technol., 201 (1-2), 371-383 (2006).
4. F.C. Walsh and C. Ponce de Leon, Trans. Inst. Metal Fin., 92 (2), 83-98 (2014).
5. Y. Zhang, JOM, 67 (11), 2599-2607 (2015).
6. B.L. Bates, J.C. Witman and Y. Zhang, Mater. Manuf. Process, 31 (9), 1232-1237 (2016).
7. A.V. Put, et al., Surf. Coat. Technol., 205 (3), 717-727 (2010).
8. A. Hovestad, R.J.C.H.L. Heesen and L.J.J. Janssen, J. Appl. Electrochem., 29 (3), 331-338 (1999).
9. R. Bazzard and P.J. Boden, Trans. Inst. Met. Finish., 50 (1), 63-69 (1972).
Past project reports
1. Quarter 1 (January-March 2018): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 82 (12), 12 (September 2018); Full paper: http://short.pfonline.com/NASF18Sep1.
About the author

Dr. Ying Zhang is Professor of Mechanical Engineering at Tennessee Technological University, in Cookeville, Tennessee. She holds a B.S. in Physical Metallurgy from Yanshan University (China)(1990), an M.S. in Materials Science and Engineering from Shanghai University (China)(1993) and a Ph.D. in Materials Science and Engineering from the University of Tennessee (Knoxville)(1998). Her research interests are related to high-temperature protective coatings for gas turbine engine applications; materials synthesis via chemical vapor deposition, pack cementation and electrodeposition, and high-temperature oxidation and corrosion. She is the author of numerous papers in materials science and has mentored several Graduate and Post-Graduate scholars.
Jason C. Whitman is a Ph.D. student in the Department of Mechanical Engineering, Tennessee Technological University, in Cookeville, Tennessee. His primary work is involved with the AESF Foundation Research Project R-119, Electro-codeposition of MCrAlY Coatings for Advanced Gas Turbine Applications.
*Corresponding author:
Dr. Ying Zhang, Professor
Department of Mechanical Engineering
Tennessee Technological University
Cookeville, TN 38505-0001
Tel: (931) 372-3265
Fax: (931) 372-6340
Email: yzhang@tntech.edu
Related Content
Nanotechnology Start-up Develops Gold Plating Replacement
Ag-Nano System LLC introduces a new method of electroplating based on golden silver nanoparticles aimed at replacing gold plating used in electrical circuits.
Read MoreLiquid Chrome Vs. Chromic Acid Flake
Contemplating how to continue offering chromic acid services in an increasingly stringent regulatory world? Liquid chrome products may be the solution you’re looking for.
Read MoreHow to Maximize Nickel Plating Performance
The advantages of boric acid-free nickel plating include allowing manufacturers who utilize nickel plating to keep up the ever-changing regulatory policies and support sustainability efforts.
Read MoreProducts Finishing Reveals 2024 Qualifying Top Shops
PF reveals the qualifying shops in its annual Top Shops Benchmarking Survey — a program designed to offer shops insights into their overall performance in the industry.
Read MoreRead Next
Delivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read More











.jpg;maxWidth=300;quality=90)









