Faraday’s Law Applied to Cleaning
This paper is a re-publication of the the 2nd William Blum Lecture, presented at the 47th AES Annual Convention in Los Angeles by Dr. A. Kenneth Graham, 1959 AES Scientific Achievement Award recipient. While Faraday's Law had long been applied to electrodeposition processes, Dr. Graham took it a step further, considering its usage in the electrocleaning of various metal substrates in terms of electrical charge passed during the cleaning operation.
by
Dr. A. Kenneth Graham
Recipient of the 1959 AES Scientific Achievement Award
Editor’s Note: Originally published as A.K. Graham, Annual Technical Proceedings of the American Electroplater’s Society, 47, 41-44 (1960), this article is a re-publication of the 2nd William Blum Lecture, presented at the 47th AES Annual Convention in Los Angeles, California, on July 25, 1960. A printable PDF version is available by clicking HERE.
Introduction and background
“What is Faraday's Law?" That was the only question asked me by the plating foreman of the Welsbach Company in 1921 when the superintendent introduced me and inquired if there might be a job available for me. At the time, I had my BS degree in chemical engineering and two years’ experience in industry, but recognized the practical value of working over the tanks under an experienced plater. The superintendent agreed to give me the opportunity to apply for such a job without disclosing my background. I got the job by answering the question - "What is Faraday's Law?"
All of you appreciate that Faraday's two Laws are the basis of all plating and, in fact, of the entire Electrochemical Industry. In the years since the last war, we have learned that they can be applied with great advantage to cleaning as well as plating. It is about their application to cleaning that I wish to speak today.
Cleaning of the common basis metals for present-day decorative and protective plating applications has been developed to the point where the conscientious plater can apply the available information and do an acceptable job. However, the present-day plating applications in other areas, the so-called engineering applications, are presenting an ever increasing array of cleaning and plating problems. One industry requires electrodeposited gold, silver or alloy coating on beryllium copper, bronze or brass tape or wire. Coatings of buffed nickel over buffed nickel with or without a final chromium coating have been used to overcome cavitation failure of diesel cylinder liners and to obtain improved corrosion resistance in various applications. Many varieties of stainless steel alloys must be adherently plated with various metals to meet special service requirements. The extremely passive materials, such as Stellite, Inconel, Hastalloy, and Carballoy sometimes require adherent plated coatings for some applications. Sometimes the more exotic metals, such as niobium, titanium, zirconium, uranium, beryllium and molybdenum, require adherent plated coatings for various reasons. It is reasonable to assume that such problems will increase in the future.
Such engineering plating applications will only be successful if one is able to develop cleaning cycles that will permit one to deposit adherent metal coatings. To accomplish this one must remove oxide or other surface films and then keep the basis metal surface activated until the plated coating can be applied. The chemistry of some of the basis metals involved render this extremely difficult. There is much yet to be learned about these matters.
The conventional approach to solving such a problem is to first remove organic soils by suitable degreasing means, including an electrocleaning treatment. Whether the latter should be anodic or cathodic is not always clear. In any event, the commonly recommended conditions for a proprietary cleaner such as concentration, temperature, current density and time, are usually employed. Some acid treatment with or without the use of current is then chosen to etch and activate the surface and the commonly recommended conditions of bath composition, concentration, temperature, time and current, if any, are precisely followed. In some cases, if allowable, the Wood's type of nickel chloride strike is finally used under the conditions recommended. If these procedures then do not give the desired results, we really do have a problem.
Under such circumstances, I have found it most helpful to simply apply Faraday's Laws to the electrolytic treatments. I therefore trust you will bear with me if I briefly discuss these Laws in an elementary fashion as applied to cleaning.
According to Faraday's first Law, the amount of chemical change produced by an electric current flowing through an electrolyte is proportional to the quantity of electricity. The quantity of electricity is the product of the current flowing times the time. The unit of quantity, a coulomb, is one ampere flowing for one second. Ten amperes flowing for six seconds will cause the same chemical change as one ampere for sixty seconds, both being the same quantity of electricity, 60 coulombs or one ampere minute. In either case, the same amount of hydrogen will be liberated at a metal surface in cathodic cleaning. If one doubles the quantity of electricity, the amount of hydrogen liberated also will be doubled. Whatever benefit may be derived from the liberation of hydrogen in cathodic cleaning may therefore be varied by applying this concept.
According to Faraday's second Law, the amounts of different substances liberated by a given quantity of electricity are proportional to their chemical equivalent weights. Stated another way, 96,500 coulombs or one Faraday of electricity will liberate one equivalent weight or one gram of hydrogen at the cathode and one equivalent weight or eight grams of oxygen at the anode in alkaline electrocleaning. Therefore, the effect of the oxygen liberated in anodic cleaning, whether beneficial or otherwise, can also be varied quantitatively by applying this Law.
According to Avogadro, a gram molecular weight of any gas occupies the same volume at the same temperature and pressure. Since the equivalent or combining weight of hydrogen is one-half its molecular weight, but that of oxygen is only one-fourth its molecular weight, then the volume of hydrogen liberated at the cathode in alkaline electrocleaning is twice the volume of oxygen liberated at the anode. Thus this well-known volume relationship follows directly from Faraday's Laws. Also, the greater volume of hydrogen liberated at the cathode led to the preferred use of cathodic electrocleaning in the early days.
In many cases the scrubbing action of the volume of gas liberated at the surface of the metal in alkaline electrocleaning is of secondary importance to the nature of related chemical reactions and this depends upon the reactions of hydrogen and oxygen at the electrode surfaces. In our opinion, the resulting adhesion of the electrodeposited metal coating is the most important factor, both as an indication of a properly cleaned surface and as a means of insuring quality of the plated coating. Experience has shown that good adhesion is favored by anodic cleaning of the common ferrous metals. Cathodic cleaning is usually preferred for nickel. Copper or zinc can be cleaned either way for good adhesion, but anodic cleaning is most commonly used to avoid deposition of films. Lead is cathodically cleaned to avoid etching and staining.
The cleaning of many metals prior to plating as practiced today frequently involves various pretreatments in combination with both electrolyte alkaline and acid steps. These electrolytic treatments are usually limited to not over two minutes and often to one minute or less. The tank sizes and conveyor chain speed frequently determines this. Also, the current density is either limited to that obtainable at the voltage of the current source available or is purposely restricted because of the sensitivity of a particular metal surface with respect to etching or staining. This is especially true for decorative bright-plated finishes. Thus the quantity of electricity, the product of the time multiplied by the amperes flowing, is thereby limited and the application of Faraday's Laws, in any real sense, has been disregarded as far as cleaning is concerned.
Of course this is not so with plating. One Faraday or 96,500 coulombs of electricity will deposit one chemical equivalent or combining weight of any metal at 100 per cent cathode efficiency. We routinely refer to the Table of Electrochemical Equivalents and Related Data to find the ampere minutes required to deposit any metal to a coating thickness of one mil per square foot. From this we determine the plating time required at a given current density to deposit any thickness of metal or vice versa. We still design and control our plating operations to obtain a given plating time at a controlled average current density to obtain a controlled average weight of metal coating. We also make allowance for the efficiency of the plating process and for variations in current and metal distribution with the design of the part being plated. All this is strictly in accordance with Faraday's Laws.
We also know that Faraday's Laws apply to the performance of soluble anodes in plating and that the metal plated out at the cathode is substantially all supplied by metal dissolving at the anode in properly controlled processes.
To apply Faraday's Laws to cleaning is much more difficult and one might ask, "Why bother?" It is more difficult because we are obliged to remove so many different types of soil, and the term soil is used here in the broadest sense. The surface chemistry of the basis metal itself cannot be defined. As many of you well know who chromium plate nickel, the surface chemistry of a nickel coating immediately after plating is different than one that has just been buffed and both will be different after exposure to air for 24 hours. The surface chemistry also varies with each metal and its metallurgical history. One therefore cannot determine the chemical equivalent weight of the combination of soils, oxides and metal surface films and relate it to a given quantity of electricity in cleaning. Our only recourse is to apply the quantitative concepts of Faraday blindly. Increasing the current density and/or the time will quantitatively increase the hydrogen and oxygen liberated and the chemical reactions resulting at both anode and cathode in electrocleaning treatments, even though the reactions are undefined. By so doing we can accomplish results in cleaning and plating that can be guaranteed and not left to chance. This is especially true with respect to the so-called engineering applications, many of which require plating upon the more difficult or unusual basis metals.
Mr. F. W. Stockton, formerly of the Standard Steel Spring Co., was the first, to our knowledge, to emphasize the importance of Faraday's Laws in electrocleaning. He observed that cathodic cleaning of steel prior to nickel plating gave very poor adhesion, compared to anodic cleaning. He then showed, if cathodic cleaning was first used, the adverse effect of this treatment on adhesion could be overcome by following with anodic cleaning, using at least the same quantity of ampere minutes per square foot and preferably more.
We have extended this application of Faraday's Laws by increasing time and/or current density of both the electrolytic alkaline and acid treatments in developing cleaning cycles for specific plating applications. Each cycle so developed must be shown by test to meet the required specifications, especially the adhesion, before being used in production. Then by controlling the production cycle steps, as established by this procedure, the quality of the plated product can be assured. A few illustrations of how this has been applied may be of interest.
Plating chemical equipment
The late Carl Heussner used 18 cleaning steps including rinses for the first atomic energy program (the Manhattan Project) in the preparation of steel equipment for nickel plating. He naturally included every favorable treatment step that had been recommended in the literature in order not only to meet the corrosion and adhesion tests that were specified, but in the hope that the quality of nickel plating so produced would prove satisfactory for the intended service. Fortunately the plated nickel coatings performed successfully. Otherwise solid wrought nickel would have been required and this program alone would have consumed the available output of nickel in America for two years.1 Of course, no such quantity was available, so if plating had failed, we might not have had the atom bomb.
The equipment programs that followed the war were no longer on a crash basis and money was no longer being spent on an emergency basis. It was important therefore to limit the number of preplating steps to a minimum in order to reduce plant investments. Fortunately we had the time to investigate this. By applying the quantitative concept of Faraday in developing the cleaning cycle we were able to meet the nickel plating specification for both the adhesion and hot water porosity rating with a cycle of only four steps, two of which were rinses.2 (See Table 1.)
We knew that anodic alkaline and anodic sulfuric acid treatments favored the adhesion of nickel to mild steel. We did not know what quantity of electricity in these treatments was required to meet the specifications or, in fact, whether some further cycle variations would be required. We increased the quantity of current stepwise in both the anodic alkaline and acid treatments and ultimately found that with a minimum of 300 A-min/ft2 in both treatments we got perfect adhesion. The non-silicated proprietary cleaner that had originally been specified was operated at a concentration of 10-12 oz/gal and a temperature of 190-200°F. The anodic acid 50 per cent by volume sulfuric acid was operated at a temperature not in excess of 85°F. A minimum current density of 50 A-min/ft2 and six minutes treatment time was used in both treatments.
Table 1 - Cleaning cycles for nickel plating.*
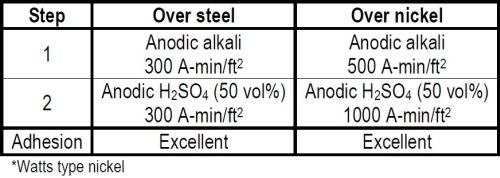
It is conservatively estimated that over three million square feet of surface were plated to an average of 5-7 mil of nickel without a single failure due to adhesion. When a piece of some 700 square feet of surface failed to pass the hot water porosity test occasionally, the defective areas were wire brushed. The piece was then given the same cleaning treatments with minor modification prior to plating additional nickel over the initial coating. No adhesion failure between the two nickel coatings was ever experienced.
In practice, the anodic alkaline electrolytic treatment was sometimes increased to as much as 500 A-min/ft2. The anodic acid treatment was sometimes increased to 1000 A-min/ft2. This was done to compensate for a particularly bad lot of steel or as a further factor of safety, especially when plating nickel over nickel. In the latter case we were always careful to use more ampere minutes per square foot in the acid treatment than in the anodic alkaline treatment, since the latter alone adversely affects the adhesion of nickel over nickel.
One must realize that this application of plating was for corrosion resistance in a chemical environment and involved the use of heavy nickel coatings. A bright appearance was of no importance. Therefore etching resulting from the long anodic acid treatment could be used to advantage. The problem is quite different when plating nickel over nickel for a decorative application, as discussed in the next example, where the coatings are thinner and luster is so important.
Plating nickel on buffed nickel
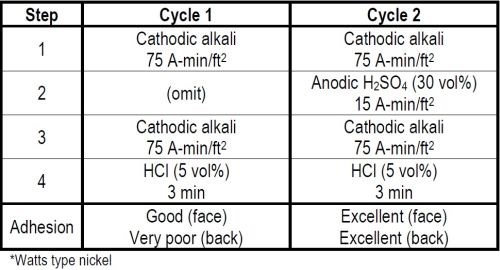
In 1947 the International Nickel Co. wanted to have some steel panels plated with double nickel coatings to be included in the outdoor exposure program of Committee B-8 of ASTM, now known as Program No. 1. The lot of panels requiring a first coating of buffed Watts type nickel, followed by a second coating of the same nickel, presented a problem. One had to clean and activate the first buffed nickel surface so that the buffed surface would not be destroyed, but at the same time would insure adequate adhesion of the second nickel coating. We knew cathodic alkali cleaning and a strong hydrochloric acid dip would favor activation of the buffed nickel surface and adhesion of the second nickel coating. We did not know the quantity of current required to accomplish this. In our laboratory evaluation of the cycle we kept increasing the ampere minutes of cathodic cleaning until as shown in Cycle 1 of Table 2 with two separate treatments of 75 A-min/ft2 each, followed by a three-minute dip in 50 volume per cent hydrochloric acid, we obtained good adhesion of nickel on the buffed nickel surface. The adhesion on the back of the test panel where the steel had only been rough-polished and the first nickel coat was unbuffed was still very poor. By then inserting between the two cathodic cleaning steps a 15 A-min/ft2 anodic etch in 30 per cent by weight sulfuric acid as shown in Cycle 2, we obtained excellent adhesion of the second nickel coating on both the front and back of a test panel. By thus applying the quantitative concept of Faraday's Laws to cleaning, we developed a satisfactory cycle and it is now a matter of record that the panels so prepared performed well when tested outdoors.
It is conservatively estimated that over three million square feet of surface were plated to an average of 5-7 mil of nickel without a single failure due to adhesion. When a piece of some 700 square feet of surface failed to pass the hot water porosity test occasionally, the defective areas were wire brushed. The piece was then given the same cleaning treatments with minor modification prior to plating additional nickel over the initial coating. No adhesion failure between the two nickel coatings was ever experienced.
In practice, the anodic alkaline electrolytic treatment was sometimes increased to as much as 500 A-min/ft2. The anodic acid treatment was sometimes increased to 1000 A-min/ft2. This was done to compensate for a particularly bad lot of steel or as a further factor of safety, especially when plating nickel over nickel. In the latter case we were always careful to use more ampere minutes per square foot in the acid treatment than in the anodic alkaline treatment, since the latter alone adversely affects the adhesion of nickel over nickel.
One must realize that this application of plating was for corrosion resistance in a chemical environment and involved the use of heavy nickel coatings. A bright appearance was of no importance. Therefore etching resulting from the long anodic acid treatment could be used to advantage. The problem is quite different when plating nickel over nickel for a decorative application, as discussed in the next example, where the coatings are thinner and luster is so important.
Plating nickel on buffed nickel
In 1947 the International Nickel Co. wanted to have some steel panels plated with double nickel coatings to be included in the outdoor exposure program of Committee B-8 of ASTM, now known as Program No. 1. The lot of panels requiring a first coating of buffed Watts type nickel, followed by a second coating of the same nickel, presented a problem. One had to clean and activate the first buffed nickel surface so that the buffed surface would not be destroyed, but at the same time would insure adequate adhesion of the second nickel coating. We knew cathodic alkali cleaning and a strong hydrochloric acid dip would favor activation of the buffed nickel surface and adhesion of the second nickel coating. We did not know the quantity of current required to accomplish this. In our laboratory evaluation of the cycle we kept increasing the ampere minutes of cathodic cleaning until as shown in Cycle 1 of Table 2 with two separate treatments of 75 A-min/ft2 each, followed by a three-minute dip in 50 volume per cent hydrochloric acid, we obtained good adhesion of nickel on the buffed nickel surface. The adhesion on the back of the test panel where the steel had only been rough-polished and the first nickel coat was unbuffed was still very poor. By then inserting between the two cathodic cleaning steps a 15 A-min/ft2 anodic etch in 30 per cent by weight sulfuric acid as shown in Cycle 2, we obtained excellent adhesion of the second nickel coating on both the front and back of a test panel. By thus applying the quantitative concept of Faraday's Laws to cleaning, we developed a satisfactory cycle and it is now a matter of record that the panels so prepared performed well when tested outdoors.
Table 2 - Cleaning cycles for nickel plating over buffed nickel.*
Nickel plating over nickel reruns
Defective nickel-chromium plated steel parts, such as bumpers, are frequently salvaged by first stripping the chromium, then polishing out the defects and re-nickel plating. Various cycles are used depending to a large degree upon the treatments available in a given plating installation. The following illustrates how Faraday's Laws were applied in one case for evaluating a cleaning cycle for this purpose.
Table 3 - Cleaning cycles for nickel plating over polished nickel.*
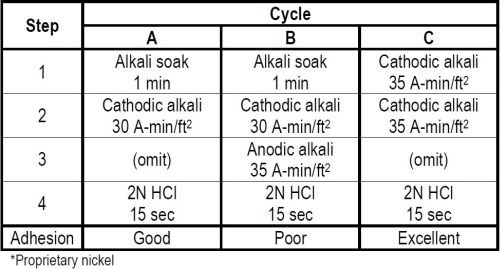
Two cleaning cycles A and B in Table 3 were first evaluated. Omitting any reference to rinsing in between treatments, both cycles included a one-minute alkali soak and 30 A-min/ft2 of cathodic electrocleaning with a 15 second 2N hydrochloric acid dip prior to nickel plating. The only difference between the two cycles was that B had in addition a 35 A-min/ft2 anodic electrocleaning step inserted ahead of the acid dip. Cycle A therefore had but 30 A-min/ft2 cathodic cleaning while Cycle B had a total of 65 A-min/ft2 of electrocleaning, the last 54 per cent of which was anodic. In spite of this additional electrocleaning adhesion between the first and second nickel coatings when using Cycle B was rated poor while A was rated good.
A third Cycle C was then evaluated in which the first two treatment steps were cathodic electrocleaning for a combined total of 70 A-min/ft2. This was followed by the same 15 second 2N hydrochloric acid dip prior to nickel plating. Cycle C gave excellent adhesion between the two nickel coatings compared to Cycle A which only rated good. This difference was obtained by substituting a cathodic electrocleaner for the alkali soak in step 1 of Cycle A, and thereby increasing the quantity of cathodic cleaning from 30 to 70 A-min/ft2.
Plating bimetal surfaces
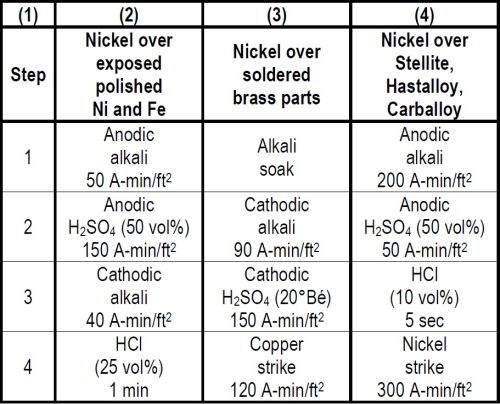
When plating reruns of nickel plated steel parts a complication arises if, in removing the nickel coating defects by polishing, steel areas are exposed, because optimum adhesion on steel is favored by anodic treatments while cathodic treatments are best for nickel. A good compromise involves the use of an anodic electrocleaning treatment followed by an anodic acid treatment, a cathodic electrocleaning treatment and a hydrochloric acid dip as shown in Column 2 of Table 4. The last three treatments all activate the nickel, thus favoring adhesion when plated. The cathodic electrocleaning which is unfavorable to steel should preferably be with a less quantity of current than the anodic electrocleaning. The anodic acid treatment which is beneficial to adhesion on both nickel and steel should always employ a greater quantity of current, than either of the alkaline electrocleaning treatments. The required ampere minutes per square foot for each electrolytic treatment should be determined by test to obtain optimum adhesion.
There are many other cases of plating over two different metals on one object in which difficulty is experienced. One case where Faraday's Laws were applied involved the plating of brass parts with soldered joints. The cleaning cycle that proved most successful as shown in Column 3 of Table 4 consisted of four steps, namely, a soak cleaner, 90 A-min/ft2 in a cathodic electrocleaner, 195 A-min/ft2 in a 20°Bé cathodic sulfuric acid treatment, and 120 A-min/ft2 in a copper strike. It is necessary to use sufficient ampere minutes per square foot in the electrolytic treatments to reduce the oxides formed in soldering in order to get adherent plated coatings. The appearance of the surface after the acid treatment may be far from pleasing, but this in no way impairs the appearance or quality of plated coatings which may be applied.
Plating Stellite and Hastalloy C
In one engineering application requiring adherent nickel plating on Stellite, Faraday's Laws were again applied to advantage in the development of the preparatory cleaning cycle as shown in Column 4 of Table 4. The desired results were finally obtained with a cycle involving treatments of 200 A-min/ft2 of anodic electrocleaning, 50 A-min/ft2 of anodic 50 volume per cent sulfuric acid, a 5 second dip in 10 volume per cent hydrochloric acid and a Wood's nickel chloride strike of 300 A-min/ft2 prior to plating.
This same cycle also was used successfully for adherently plating some Hastalloy C and Carballoy parts.
Table 4 - Cleaning cycles for plating
Plating the “exotic” metals
A qualifying word is in order with respect to cleaning the exotic metals prior to plating. Their chemistry is so different from the more common basis metals that other treatments, specifically for a given metal, may have to be added. This is the area in which much remains to be learned. However, to the extent that electrolytic treatments are involved, the application of Faraday's Laws has proven fruitful.
In conclusion, may we summarize as follows:
Assuming the removal of the varying amounts of many different organic soils in preliminary cleaning steps, the subsequent electrolytic cleaning and activating treatments determine both the adhesion and quality of the plated coating.
There are three electrolytic treatments, building blocks so to speak, commonly used in developing a successful cycle:
- Electrolytic alkali cleaning.
- An acid treatment, frequently electrolytic.
- A strike (usually Wood's nickel chloride strike), a cathodic treatment, if allowable and necessary.
We would discourage the practice of simply copying specific treatments or a combination of steps as reported in the literature when searching for a cycle to perform a difficult job of cleaning before plating. One very good reason is the fact that just changing a proprietary cleaner may change the results. But then, there are many other reasons.
On the basis of our success, we recommend instead the application of Faraday's Laws to all the electrolytic treatment steps in the development of any cycle and to the degree necessary to attain the desired adhesion and quality.
Only to the extent to which this is practiced can one learn to appreciate the full significance of Faraday's Laws as applied to cleaning before plating.
References
1. W.W. Stout, "Secret," Chrysler Corporation, Detroit (1947).
2. F.K. Savage, A. K. Graham and E. P. Strothman, Materials and Methods, August 1952.
3. A.K. Graham, Trans. Inst. Met. Finishing, 31, 259-266 (1954).
About Dr. A. Kenneth Graham (from the biography printed in 1959 at the time of his receiving the AES Scientific Achievement Award)
Dr. A. Kenneth Graham was born in Philadelphia, D
He then worked in the research department of the Scovill Mfg. Co., Waterbury, Conn, for two years. During this time he met and worked with George B. Hogaboom. This was the beginning of a life-long friendship. It was through this association that Dr. Graham became interested in electroplating and decided to specialize in this field.
Since there was no modern text on plating until Blum and Hogaboom published their book in 1923, Dr. Graham decided the best way to learn about plating was to work at it. This he did as a plater's helper from October 1920 until July 1921 - first at the Hartford Sterling Co., Philadelphia, and then at the Welsbach Co., Gloucester, N.J.
The summer of 1921, Dr. Graham studied metallography at Columbia University. This was followed by five years in the graduate school of the University of Pennsylvania, during which time he served as Instructor in Chemistry and completed work leading to a technical degree of Ch.E. and graduate degrees of M.S. and Ph.D.
After a six-month bout with an ulcer, Dr. Graham delivered his first paper before the AES at the convention in Newark in June 1926, for which he later received a medal award.
He then joined the Hanson-Van Winkle Company of Newark, N.J. and became associated with George Hogaboom for the second time. He installed a laboratory at Newark and later at Matawan, N.J., when the Hanson Company joined with Munning. During this period the simplified methods of analysis for plating baths were developed, the course for platers was first offered, the standard solutions for analyses were made available for platers, and the zinc-aluminum anode, which later became patented, was developed.
Starting in the fall of 1928, Dr. Graham held the following positions:
- University of Pennsylvania, Philadelphia, Pa., Instructor and Assistant Professor in Chemical Engineering, September 1928 to February 1937.
- Kenneth Graham & Associates, Jenkintown, Pa., Consulting Engineer, July 1936 to June 1942.
- War Production Board, Washington, D. C., Plating Consultant and Deputy Chief, Products Branch, Conservation Division, June 1942 to June 1943.
- Houdaille-Hershey Corp., Decatur, Ill., Director of Research and Development, Garfield Division, June 1943 to August 1944.
- Graham, Crowley & Associates, Inc., (later becoming Graham, Savage & Associates, Inc.), Jenkintown, Pa., President, September 1944 to date [as of this writing in June 1959 - Ed.].
Dr. Graham joined the Newark Branch of the AES in 1926. In 1928, he transferred to the Philadelphia Branch. He organized and taught the first chemistry class for the Philadelphia Branch of the AES. He has won three gold medal awards for papers from the AES. He served as Executive Secretary of the Society from 1945 to 1951, during which time the monthly publication was changed from the small size Monthly Review to the present format of Plating. He was elected an Honorary Member of the Philadelphia Branch in 1957.
He is also a member of the Institute of Metal Finishing, ASTM, ACS, AIChE, Association of Consulting Chemists and Chemical Engineers, Inc., and The Electrochemical Society. He has been active on committees of most of these societies and served as manager and vice president of The Electrochemical Society for eleven years.
He has published over forty technical papers, obtained several patents, contributed chapters to Modern Electroplating by Gray, the ACS Monograph on Copper by Butts, and the Electroplating Engineering Handbook, for which he also served as Editor-in-Chief.
Related Content
Curing Oven Basics
Simply heating up the substrate does not cure the coating. There are many variables to consider when choosing the best cure oven for your application...
Read MoreHow to Address Declining Powder Coating Coverage Over Time
Fine particles from reclaim could be to blame for powder coating problems that emerge over time. Avoid problems by keeping hooks clean, maintaining guns and using reclaim powder quickly to avoid accumulation of fines.
Read MoreA Chromium Plating Overview
An overview of decorative and hard chromium electroplating processes.
Read MoreTop Reasons to Switch to a Better Cleaning Fluid
Venesia Hurtubise from MicroCare says switching to the new modern cleaning fluids will have a positive impact on your cleaning process.
Read MoreRead Next
A ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read More


















.jpg;maxWidth=300;quality=90)









