Impact of REACH Regulation on the Global Finishing Market
This paper is a peer-reviewed and edited version of a presentation delivered at NASF SUR/FIN 2012 in Las Vegas, Nev., on June 12, 2012.
By Lionel Thiery, Coventya Holding SAS, and Dr. Klaus Wojczykowski, Coventya, Inc.
Editor's Note: This paper is a peer-reviewed and edited version of a presentation delivered at NASF SUR/FIN 2012 in Las Vegas, Nev., on June 12, 2012.
ABSTRACT
REACH regulation 1907/2006 entered into force on June 1, 2007. It was adopted to enhance protection of human health and the environment from risks posed by certain chemicals, while at the same time increasing the competitiveness of the EU chemicals industry. To this date, producers and/ or importers of substances register the most hazardous substances and will continue to do so until 2018. Downstream users like platers will now face this requirement and all segments of the general metal finishing market will be governed by these regulations. Alternative treatments compliant with REACH regulation must also be foreseen. Chemicals used in the future must not be present in the list of SVHC (Substances of Very High Concern) and possibly non-CMR. For this reason, chemical suppliers must ensure that research and development focuses on bringing alternative sustainable technologies to the market. This paper will show how globally operating chemical suppliers react to constraints and opportunities evolving out of the new legislations by adapting R&D and business strategies.
Keywords: REACH impact, Cr(III) passivates, cobalt salts, cadmium substitutes, zinc-manganese deposits, gold-copper-indium alloy deposits
Introduction
The REACH regulation1 was entered into force on June 1, 2007. It was adopted to enhance the protection of human health and the environment from risks posed by certain chemicals, while at the same time increasing the competitiveness of the EU chemicals industry.
To this date, producers and/or importers of substances register the most hazardous substances and will continue to do so until 2018. Downstream users such as formulators and platers face this requirement and all segments of the general metal finishing market will be governed by these regulations. Alternative treatments which are compliant with REACH regulation must also be foreseen.
Chemicals used in the future must not be present in the list of SVHC (Substances of Very High Concern) and possibly non-CMR. For this reason, chemical suppliers must ensure that research and development focuses on bringing alternative sustainable technologies to the market. This paper will show how globally operating chemical suppliers react to constraints and opportunities evolving out of the new legislations by adapting R&D and business strategies.
REACH impact on the supply chain2
As shown in Fig. 1, the REACH regulation has an impact on the global supply chain, as manufacturers and importers of substances as well as formulators and downstream users like job platers are impacted at different levels.
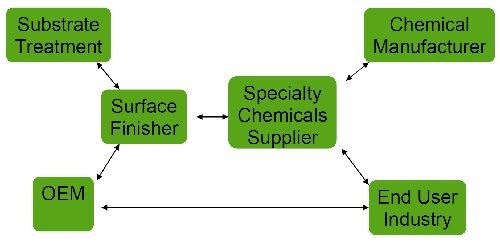
Figure 1 - Impact of REACH on the global supply chain.
The registration process has an impact first of all on the manufacturer or importer in the European Union of a substance manufactured or imported in quantities over 1 T/yr. Thanks to the pre-registration process, phase-in substances could be pre-registered until December 2008, allowing the registration to be postponed until June 1, 2013 or June 1, 2018, depending on the considered tonnage band.
Unfortunately, for non-phase-in substances, no delay in registration is allowed and they must be registered immediately. The registration dossier includes a technical dossier and a Chemical Safety Report if the registrant produces or imports the substance in quantities over 10 T/yr.
Downstream users are impacted in the registration phase. They have to follow the instructions in the safety data sheets they receive. If their use is not covered by an exposure scenario, they can communicate with their supplier with the aim of having their use covered by an exposure scenario or they may need to develop their own chemical safety report. If the registered substance is listed as SVHC and classified in Annex XIV (authorization list), an authorization for the specific use must be requested to the ECHA.
The authorization procedure aims to ensure that the risks from Substances of Very High Concern are properly controlled and that these substances are progressively replaced by suitable alternatives while ensuring the smooth functioning of the EU internal market.
Substances with the following hazard properties may be identified as Substances of Very High Concern (SVHCs):
-
Substances meeting the criteria for classification as carcinogenic, mutagenic or toxic for reproduction category 1A or 1B in accordance with Commission Regulation (EC) No 1272/2008 (CMR substances).
- Substances which are persistent, bioaccumulative and toxic (PBT) or very persistent and very bioaccumulative (vPvB) according to REACH (Annex XIII).
Substances identified on a case-by-case basis, for which there is scientific evidence of probable serious effects that cause an equivalent level of concern as with CMR or PBT/vPvB substances. After a two-step regulatory process (Fig. 2), SVHCs may be included in the Authorization List and become subject to authorization. These substances cannot be placed on the market or used after a given date, unless an authorization is granted for their specific use, or the use is exempted from authorization. Manufacturers, importers or downstream users of a substance on the Authorization List can apply for authorization.
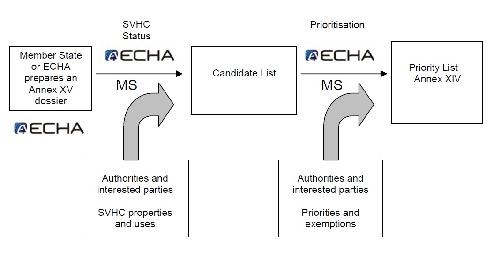
Figure 2 - Listing and authorization process.
Restrictions are a tool to protect human health and the environment from unacceptable risks posed by chemicals. Restrictions may limit or ban the manufacture, commercialization or use of a substance. A restriction applies to any substance on its own, in a mixture or in an article, including those that do not require registration. It can also apply to imports. A Member State, or ECHA on request of the European Commission, can propose restrictions if they find that the risks need to be addressed on a Community wide basis.
Impact on formulators of chemical specialties
Registration process
Companies have the responsibility of collecting information on the properties and the uses of substances that they manufacture or import at or above one tonne per year. They also have to make an assessment of the hazards and potential risks presented by the substance.
This information is communicated to ECHA through a registration dossier containing the hazard information and, where relevant, an assessment of the risks that the use of the substance may pose and how these risks should be controlled.
Registration is based on the "one substance, one registration" principle. This means that manufacturers and importers of the same substance are obliged to submit their registration jointly. Besides individual substances generally registered by the manufacturer or importer, formulators make use of organic reaction products acting on the grain formation during the reduction process at the cathode. These organic syntheses will result in new substances. Most of them fall into the polymer definition according to REACH and are therefore exempt from registration.
Nevertheless, some of them do not fall under this definition and have to undergo the registration process.
The costs associated with the registration of a new molecule are significant. Besides the registration fee, there are analytical tests which include physico-chemical tests, toxicological and ecotoxicological studies representing the major part of the associated costs. They will also depend on the tonnage band for which registration is applied. As an example, registration costs for a new substance can be over 400,000 € (approximately $520,000 as of November, 2012) for a tonnage band less than 100 T/year.
The immediate effect is to limit the development of new molecules for the surface treatment industry. Many additives used in the electroplating industry result in synthesis of organic molecules.
Polymers are exempted from REACH and therefore these substances will be increasingly favored by specialty chemicals suppliers.
Authorization process
The candidate list of substances for authorization was updated on December 19, 2011. ECHA has developed a paper presenting its approach for prioritizing, pursuant to Article 583 of the REACH regulation, substances for inclusion in Annex XIV.2 On the basis of this approach, ECHA has prioritized the following thirteen substances from the Candidate List of Substances of Very High Concern for inclusion in Annex XIV, shown in Table 1.
Table 1 - ECHA's approach for prioritizing substances for inclusion in Annex XIV.
|
Group |
|
Substance name |
EC |
|
|
1 |
Trichlorethylene |
201-167-4 |
|
Chromium (VI) compounds |
2 |
Chromium trioxide |
215-607-8 |
|
3 |
Acids generated from chromium trioxide and their oligomers; Group containing: |
|
|
|
|
Chromic acid, Dichromic acid Oligomers of chromic acid and dichromic acid |
231-805-5 236-881-5 Not yet assigned |
|
|
4 |
Sodium dichromate |
234-334-2 |
|
|
5 |
Potassium dichromate |
231-906-6 |
|
|
6 |
Ammonium dichromate |
232-143-1 |
|
|
7 |
Potassium chromate |
232-140-5 |
|
|
8 |
Sodium chromate |
231-889-5 |
|
|
Cobalt (II) compounds |
9 |
Cobalt(II) sulphate |
233-334-2 |
|
10 |
Cobalt dichloride |
231-589-4 |
|
|
11 |
Cobalt(II) dinitrate |
233-402-1 |
|
|
12 |
Cobalt(II) carbonate |
208-169-4 |
|
|
13 |
Cobalt(II) diacetate |
200-755-8 |
The recommended latest application dates are based on the assumption that the substances listed in this recommendation will be included in Annex XIV in February 2013.
As several substances listed above are commonly used in the surface treatment industry, it indicates that a considerable number of processes used in the field of surface treatment will be impacted by this authorization process. Besides the requests for authorization, an obvious task for specialty chemicals suppliers is to work on the substitution of these hazardous chemicals in their processes.
An analysis of the areas of concern and the possible alternatives will now be investigated.
Chromic acid and dichromate salts
The inclusion of these substances in the Annex XIV will have a strong impact on the protective and decorative markets. Although in the decorative plating area, alternatives to Cr(VI) are available with the use of Cr(III), plating from trivalent chromium electrolytes does not yet offer the same performance in corrosion tests such as the CASS test (Copper Acetic Salt Spray Test) according to ISO 9227.
Impact on the POP sector. In the plating on plastics (POP) sector, exterior parts are exposed to harsh environments. Chromium deposits made from Cr(III) chemistry offer good corrosion resistance to Russian mud (CaCl2) but generally poor resistance to CASS testing. Some alternatives have been developed, applying an additional top layer on the trivalent chromium deposit, either chemically or electrolytically. A drawback associated with these solutions is the need for an additional treatment in the plating sequence and of course this final layer has to be free of Cr(VI).
Another possible alternative is to modify the layers under the trivalent chromium to offer a better combination with the final Cr(III) layer.
The development of a nickel-alloyed underlayer beneath the trivalent chromium improved the corrosion resistance in the CASS test, as shown in Table 2 and Figs. 3 and 4.
Table 2 - Evolution of corrosion resistance (ISO 9227) of various systems on plastic parts; rating according to ISO 10289.
|
Type of parts |
Samples |
Cass exposure |
CaCl2 test |
|||||
|
24 hr |
48 hr |
72 hr |
96 hr |
120 hr |
144 hr |
|||
|
Panels |
Duplex Ni (20-25µm) + Microporous Ni (2-3µm) + Cr(VI) (0.3µm) |
4/4 10 |
4/4 10 |
2/4 10 2/4 9 |
3/4 8 1/4 9 |
--- |
--- |
Negative |
|
Automotive parts |
Duplex Ni (20-25µm) + Microporous Ni (2-3µm) + Cr(VI) (0.3µm) |
4/4 10 |
4/4 10 |
2/4 10 2/4 9 |
3/4 8 1/4 9 |
--- |
--- |
Negative |
|
Panels |
Duplex Ni (20-25µm) + Microporous Ni (2-3µm) + Cr(III)a (0.3µm) |
4/4 8 |
4/4 4 |
--- |
--- |
--- |
--- |
Positive |
|
Automotive parts |
Duplex Ni (20-25µm) + Microporous Ni (2-3µm) + Cr(III)a (0.3µm) |
4/4 8 |
4/4 4 |
--- |
--- |
--- |
--- |
Positive |
|
Panels |
Duplex Ni (20-25µm) + Noble Ni alloy (1.5-2.5µm) + Cr(III)a(0.3µm) |
4/4 10 |
4/4 10 |
4/4 10 |
4/4 10 |
4/4 9 |
4/4 8 |
Positive |
|
Automotive parts |
Duplex Ni (20-25µm) + Noble Ni alloy (1.5-2.5µm) + Cr(III)a(0.3µm) |
4/4 10 |
4/4 10 |
4/4 10 |
3/4 8 1/4 9 |
3/4 7 1/4 8 |
- |
Positive |
|
aTristar 300, Coventya Worldwide Headquarters, La Garenne, France. |
||||||||


Figure 3 - Microporous Ni + Cr(III) Figure 4 - Alloy Ni + Cr(III)
after 48 hr CASS test. after >96 hr CASS test.
Impact on the conversion layers for zinc and aluminum. Chromate and dichromate salts have been and are still used in the conversion layers on top of zinc, zinc alloys, cadmium and aluminum. The oxidizing properties of hexavalent chromium act to oxidize the substrate. The subsequent pH increase at the interface favors the precipitation of zinc and chromium hydroxides and oxides, creating a gel-like structure which upon drying will form a micro-cracked layer with major anticorrosion properties (Fig. 5).
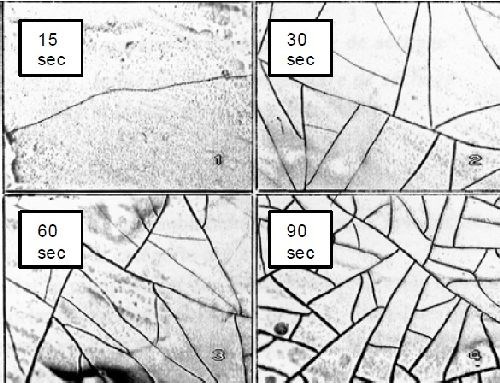
Figure 5 - Microcracked network formation of a Cr(VI) converstion layer on Zn/Fe vs. drying time.4


Figure 6 - Conversion treatment on aluminum* (L) and with optimized process (R).
*Lanthane 613.3, Coventya Holding SAS.
The ELV, RoHS and WEEE directives have restricted and almost banned the use of Cr(VI) for these applications, the preferred alternative being the use of Cr(III)-based chemistry. Nevertheless, some sectors such as the building industry and the aerospace industry are not concerned by these directives and have not yet switched to alternative solutions. The aerospace industry is particularly concerned with the substitution of Cr(VI) for conversion layers and sealing on copper-rich aluminum.5 Actual alternatives based on the combination of Cr(III) and zirconates fulfill the requirements on most of the aluminum types, but copper-rich aluminum like the 2024 T3 type still offer inconsistent results. Very recent developments of new passivates for trivalent chromium made in our laboratories offer more consistent results on laminated 2024 T3 aluminum. The conductivity and paintability properties are maintained (Fig. 6).
Further to the announcement to place CrO3 and dichromate salts in Annex XIV, some consortia like McKenna have been created in order to react and prepare the authorization dossiers and to apply for exemptions.
Cobalt salts
The Cr(III) conversion layers on zinc and zinc alloys contain a significant amount of cobalt salts. Cobalt acts as a catalyst to regulate the kinetics of conversion layer growth, but it is also incorporated into the layer, thus participating in the inhibition of corrosion.6 As described above, Co(II) salts are subject to the authorization process under REACH. The removal of cobalt from the conversion solutions results in a major loss of corrosion resistance as seen in Fig. 7.


Figure 7 - Appearance of (A) cobalt-free and (B) cobalt-containing passivates on pure zinc after 72 hr of neutral salt spray.
Co(II) salts are then progressively substituted by alternative, non-hazardous elements that are also incorporated in the layers. The incorporation of these elements enabled the same or enhanced Cr(III) layer weights to be achieved (Table 3), ensuring proper corrosion resistance of the system (Figs. 8 and 9).
Table 3 - Layer weight of cobalt vs. non-cobalt containing passivates.
|
Conversion system* |
Cr [mg/dm²] |
Co [mg/dm²] |
Si [mg/dm²] |
|
Lanthane TR 175 |
0.6 |
0.05 |
1.3 |
|
Lanthane 316 |
0.8 |
0.05 |
|
|
Lanthane 316 CF |
1.0 |
- |
- |
|
Finidip 128 CF |
0.9 |
- |
- |
|
Lanthane SI 358 |
0.9 |
- |
1.5 |
|
*Proprietary names retained for clarity; CF denotes Co-free.
|
|||

Figure 8 - AppAppearance of proprietary cobalt-free trivalent chromium passivate† on zinc-nickel (12-15% Ni) after 720 hr of neutral salt spray (L) without heat treatment and (R) with heat treatment at 120°C for 24 hr.

Figure 9 - Appearance of proprietary cobalt-free trivalent chromium passivate† on alkaline zinc after 720 hr of neutral salt spray (L) without heat treatment and (R) with heat treatment at 120°C for 24 hr.
†Finidip 128 CF Passivate, Coventya Inc.
Cadmium
Cadmium has been prohibited since the implementation of the RoHS, WEEE and ELV directives. Nevertheless, these directives concern the automotive, electronics and electrical industries. Other industries which did not previously substitute cadmium are now using a substitute process. Apart from the aerospace industry, which is considering Zn/Ni alloy with high nickel content as the most adequate alternative technology so far, some other industries such as jewelry are now impacted by the REACH 1907/2006 European regulation. Indeed, cadmium and cadmium oxides are classified as carcinogenic, aquatic acute and chronic toxic. Cadmium is mostly prohibited for use in jewelry.
In addition, cadmium and cadmium oxides are listed in the registry of current SVHC intentions. It was thus mandatory to introduce substitutes to the traditional Au/Cu/Cd alloys used in this sector.
Au/Cu/In has been found to be a good alternative to Au/Cu/Cd alloys as indium salts are not currently listed in any of the REACH lists. The use of indium alloyed with gold and copper enable one to produce colors matching the usual 1N to 3N as defined in the Swiss standard. In addition, the anticorrosion properties of an 18 carat Au/Cu/In system7,†† were found to be comparable to those of Au/Cu/Cd and fulfilled the specifications of the major end users (Table 4).
††Omegal 180 CDF Au/Cu/In Plating Process, Coventya Inc.
Table 4 - Corrosion resistance of Au/Cu/In alloy.
|
Protocol number* |
Type of test |
Standard reference |
Test duration (hr) |
Result |
|
46944 |
Cross hatch adhesion |
DIN 53151 |
0 |
Positive |
|
46945 |
Salt spray test |
ISO 9227 NSS (EN) |
96 |
Positive |
|
46946 |
Thioacetamide |
ISO 4538 (EN) |
48 |
Positive |
|
46947 |
Humidity test, 95%RH @ 50°C |
ISO 4611 (EN) |
96 |
Positive |
|
46952 |
Synthetic sweat |
NFS 80772 (EN) |
24 |
Positive |
*46944: no detachment
46945: no alteration after 96 hours of test
46946: no alteration after 48 hours of test
46947: no alteration after 96 hours of test
46952: no alteration after 24 hours of test
Boric acid
Boric acid has been included in the candidate list of SVHC since 2010/06/18 due to its toxic for reproduction characteristics. It has not been prioritized for inclusion in annex XIV. Nonetheless its substitution has to be considered. Boric acid is an excellent buffering agent used in many electroplating baths working at mild acidic pH around 4 to 5, such as acid zinc and zinc alloys or Watts nickel baths. Boric acid alternatives exist, especially for zinc baths. For Watts nickel and trivalent chromium plating baths, substitution is more difficult, since boric acid also controls deposit properties, including ductility and structure. The alternatives are presently under development.
Restrictions
As confirmed in the REACH regulation, nickel must not be used in articles which are intended to come into direct and prolonged contact with the skin. The EN 1811: 2011 standard sets the limit for nickel leaching at 0.5 µg/cm2/week. The limit of acceptance has been drastically reduced compared to the previous version of this norm. Consequently, some deposits based on the use of nickel as an alloying element no longer fulfill the requirements (Tables 5 and 6).
This is the case for Pd/Ni alloys containing 20% Ni in the alloy, and alternatives to these deposits have to be found. Pure palladium is not the real alternative, both in terms of cost and internal stress in the layer. For the same reasons described above, Pd/Co cannot be considered as an alternative to Pd/Ni since cobalt salts are even more in focus than nickel salts. Indium was found to be a good candidate for the replacement of nickel in the Pd/Ni alloyed layer. Indium is incorporated in the alloy at a percentage varying between 10 and 20%.
Table 5 - Nickel release test on Co- and Ni-free Pd alloy deposits.†††
|
Protocol number* |
Type of test |
Standard reference |
Test duration (hr) |
Result |
|
47193 |
Nickel release test |
EN 1811 (EN): 2011 |
168 |
Positive |
*47193: Nickel release < 0.5 µg/cm²/week
†††Decomet 400 Pd Alloy Plating Process, Coventya Inc.
Table 6 - Nickel release and corrosion tests on Pd/Ni alloy deposits.
|
Protocol number* |
Type of test |
Standard reference |
Test duration (hr) |
Result |
|
47193 |
Nickel release test |
EN 1811 (EN): 2011 |
168 |
Negative |
*47193: Nickel release 1.57 µg/cm²/week
Nickel salts are for the most part classified as CMR and are prone to be proposed for inclusion in the candidate list of substances for authorization. France has already proposed the inclusion of nickel salts. From 2008 thru 2011, five nickel salts were pre-selected but not listed at the ECHA level.
The Nickel Institute coordinated the actions to fight against this proposal. The major actors in the nickel world grouped together and provided evidence that nickel salts cannot be substituted at the present level of knowledge. None of the proposed alternatives fulfill the requirements of all the various industries. The last proposal for inclusion in the candidate list did not include nickel salts meaning, that at least until 2013, nickel salts should not be proposed for placement on the candidate list.
Nevertheless, alternatives to nickel still have to be foreseen. Zinc-nickel alloys with a high percentage of nickel are widely used in the automotive industry for their high performance against corrosion. Some other properties, such as contact with aluminum and reduced hydrogen embrittlement versus pure zinc, have made Zn/Ni the coating of choice for the automotive industry. It is therefore the responsibility of specialty chemicals suppliers to envisage what the alternatives to Zn/Ni could be.
The solutions must be compatible with the requirements of the automotive industry, not only in terms of corrosion resistance but also in terms of cost effectiveness. The alternatives will be submitted to corrosion tests as defined by the automotive industry, namely neutral salt spray tests, cyclic corrosion chamber tests, life cycle tests and outdoor exposure tests. Figure 9 shows the results of natural exposure of a Zn/Mn alloy deposit. This alloy7 has unique properties in outdoor exposure tests, as the manganese oxides formed during the corrosion process will inhibit further corrosion of the deposit. Nevertheless the brownish aspect of these corrosion products prevented its qualification, mainly in the automotive industry.

Figure 10 - Zn/Mn (>4% Mn) deposit after two years exposure to a seacoast atmosphere.
Conclusion
The REACH regulation is often seen as an obstacle for the chemical industry in Europe. In addition, for the surface treatment industry, such a massive regulation puts pressure on small companies which are not prepared and/or do not have the resources to face all the requirements of this regulation.
On the other hand, it can be seen as an opportunity for Europe to go in the direction of innovative coatings which are of course more respectful of human health and the environment.
References
1. Regulation (CE) No. 1907/2006 dated 18 December 2006, concerning Registration, Evaluation and Authorization of Chemical Substances.
2. L. Thiery, “SME-driven Innovation in Surface Finishing”, Greenovate, Dinner debate, Brussels, Belgium, February 1, 2011.
3. http://www.reachonline.eu/REACH/EN/REACH_EN/article58.html
4. M.P. Gigandet and L. Thiery, “Chromatation,” Les Techniques de l’Ingénieur (http://www.techniques-ingenieur.fr), M1558, 12-2004.
5. L.Thiery and F. Raulin, “Hexavalent Chromium Free Conversion Coatings for the Treatment of Aluminum,” Proc. Surfair 2008 Conference.
6. H. Sahrhage, Metalloberfläche, 66, 18-21 (2012).
7. K Wojczykowski, “New Developments in Corrosion Testing: Theory, Methods and Standards,” Proc. NASF SUR/FIN 2010, NASF, Washington, DC, 2010.
.jpg;maxWidth=600)

Related Content
A Smooth Transition from One Anodizing Process to Another
Knowing when to switch from chromic acid anodizing to thin film sulfuric acid anodizing is important. Learn about why the change should be considered and the challenges in doing so.
Read MoreProducts Finishing Reveals 2024 Qualifying Top Shops
PF reveals the qualifying shops in its annual Top Shops Benchmarking Survey — a program designed to offer shops insights into their overall performance in the industry.
Read MorePlating, Anodizing Equipment for Variety of Applications
Titan Metal Fabricators Titan Metal Fabricators, Rightech Fabrications and Optimum Anode Technologies are leading suppliers of equipment for the plating and anodizing industries.
Read MoreFinishing Systems Provider Celebrates 150 Years, Looks to Future
From humble beginnings as an Indiana-based tin shop, Koch Finishing Systems has evolved into one of the most trusted finishing equipment providers in the industry.
Read MoreRead Next
Delivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read More








.jpg;maxWidth=300;quality=90)












