Innovative Applications of Electroplating and PVD for New Material Solutions - The 54th William Blum Lecture
The following is Dr. Per Møller’s William Blum Memorial Lecture at SUR/FIN 2018, in Cleveland, Ohio on June 4, 2018, with commentary by NASF Technical Editor James H. Lindsay. It covers a variety of projects involved with surface finishing applications, including sustainable energy. Due to Dr. Møller’s serious illness, he was unable to make the journey to SUR/FIN. In his stead, his longtime professional colleague, Dr. Lars Pleth Nielsen, of the Danish Technological Institute (DTI), presented the lecture.
presented by
Dr. Lars Pleth Nielsen
for
Dr. Per Møller
Recipient of the 2017 William Blum
AES Scientific Achievement Award
Editor’s Note: The following is Dr. Per Møller’s William Blum Memorial Lecture at SUR/FIN 2018, in Cleveland, Ohio on June 4, 2018, with commentary by NASF Technical Editor James H. Lindsay. Due to Dr. Møller’s serious illness, he was unable to make the journey to SUR/FIN. In his stead, his longtime professional colleague, Dr. Lars Pleth Nielsen, of the Danish Technological Institute (DTI), presented the lecture. A printable pdf version of the presentation with commentary is available by clicking HERE.
Introductory material

Slide 1 - Title.
There was great concern about the outlook for Dr. Møller’s health, and Dr. Nielsen began the lecture by reporting on his current status (Slide 2). While the matter was very serious, the good news was that a stem cell donor had been found and the prognosis was good.

Slide 2 - Dr. Møller’s health status.
Dr. Nielsen also shared some of the published news items, both here and in Denmark, that covered the good news that Dr. Møller had received the Scientific Achievement Award for 2017 (Slides 3-4).

Slide 3 - Plaudits on the Award (1).

Slide 4 - Plaudits on the Award (2).
Dr. Nielsen was the perfect person to deliver the lecture in Dr. Møller’s stead. The two are a very close team, and have collaborated on numerous research projects, and have published numerous papers and books together (Slide 5).

Slide 5 - Long-time collaborators Møller and Nielsen.
It was noted that Dr. Møller was dedicated to the surface finishing field, his mind constantly on the lookout for examples of metal finishing performance in the field. At SUR/FIN 2015 in Rosemont, Illinois, Dr. Nielsen pointed out that the many attractions in the Chicago area (Slide 6), were superseded by Dr. Møller’s interest in examining the corrosion performance of street hardware in the city (Slide 7).
Slide 6 - Tourist activities in Chicago.

Slide 7 - Dr. Møller’s activities in Chicago.
Dr. Nielsen outlined the lecture (Slide 7), which was in essence a career retrospective of the work of this very talented man. He began the lecture by noting that “surfaces are everything,” noting that surfaces, through control of their properties, are essential to everything in our world, from the scientific technologies to mundane everyday products (Slide 8). The emphasis of the talk related to current efforts in sustainable energy made possible by electrochemical technology.

Slide 8 - Blum Lecture outline.

Slide 9 - Surfaces are everything.
Patents
Dr. Møller has been awarded numerous patents (Slides 10-15). In the patent portfolio, is a method for manufacturing implantable medical devices [Slide 11; US Patent 5,772,864 (1998)], by electroforming a prosthesis on a dissolvable mandrel. Another involves a process for electrodepositing copper wire contacts on silicon-based solar cells (Slide 12; with BP Solar; US Patent 6,881,671 (2005); European patent appl.). Another patent cites a unique slip ring, using electrodeposited rhodium for wind turbines (Slides 13-14; Vestas Wind Systems; WO/2001/061795 (2001)). Dr. Møller was instrumental in the development of a process for producing stress-free nickel and cobalt electrodeposits (EP0835335B1) for Daimler Benz Aerospace. This was the key to the use of additive layer manufacturing (ALM) of the rocket engine for the Ariane Vulcan 2 (Slide 15).
Dr. Nielsen pointed out that years of experience with innovation have given Dr. Møller the ability to motivate students and collaboration partners to develop components and devices which create meaningful solutions instead of focusing on useless patents.

Slide 10 - Selection of Møller patents.
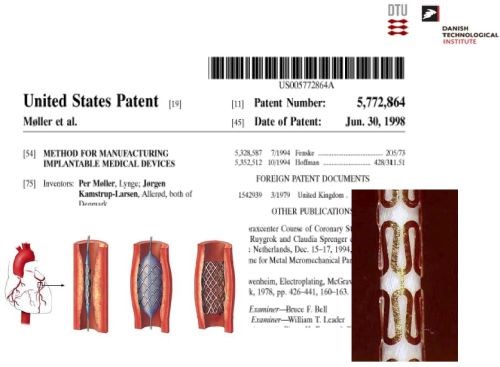
Slide 11 - Electroformed stents.

Slide 12 - Deposited metal contacts and solar cells.
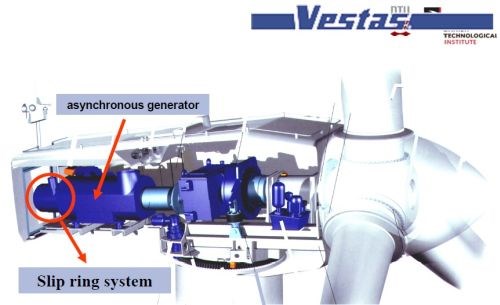
Slide 13 - Slip ring in wind turbine generator (1).

Slide 14 - Slip ring in wind turbine generator (2).

Slide 15 - Additive layer manufacturing for a rocket engine.
Published Books

Slide 16
Dr. Møller has published many papers and a number of books during his career, but the most significant one was published in 2013. Co-authored with Dr. Nielsen, the two-volume, 1240-page Advanced Surface Technology was lauded as the most comprehensive text on surface engineering technology published to date (Slides 17-19). Currently in development is another text which promises to be as important as Advanced Surface Technology - a new book on corrosion, Understanding Corrosion from an Applied Perspective (Slide 20). In addition to the information shown in Slide 20, for the chapter on corrosion types, topics will include (a) introductory material; (b) thermodynamics and the equilibrium potential; (c) corrosion potential, polarization and Pourbaix diagrams and (d) optimal material and surface selection for corrosion protection.

Slide 17 - Advanced Surface Technology (1); Covers.

Slide 18 - Advanced Surface Technology (2); Plaudits.

Slide 19 - Advanced Surface Technology (3); Contents.
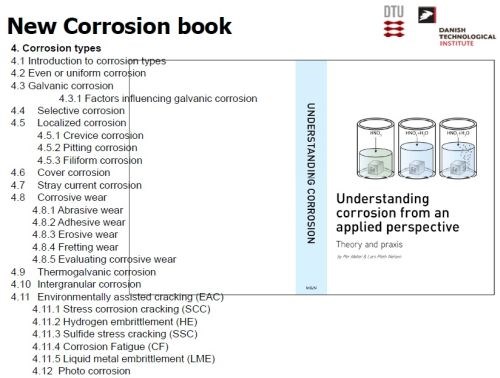
Slide 20 - New Corrosion book, Understanding Corrosion from an Applied Perspective.
Methane gas storage for renewable energy (MeGa-StoRE)
Sustainable energy is a hallmark of the power profile in Denmark. Dr. Møller’s work in this area has contributed to a program called MeGa-StoRE (Slide 21), where wind energy is converted to methane gas and stored in the country’s natural gas grid.
At present, about 40% of Denmark’s electrical supply is provided by wind turbines (Slide 22), with plans to exceed 50% by 2020-21. Wind turbine technology has advanced over the years, with the power generated by an individual unit continuing to increase (Slide 23). Denmark is a net exporter of energy when the wind is above a certain level.
At the same time, biogas is extensively used as an energy source. Consisting of methane and carbon dioxide, thermophilic biogas is produced from waste generated by farms (manure) and industrial waste products.
Using electrocatalysis via alkaline electrolysis, Dr. Møller has developed the means of converting the wind energy from turbines into hydrogen (Slide 24). In turn, the hydrogen is reacted with the carbon dioxide in biogas to produce chemically pure methane. Virtually all of the CO2 can be converted, and the methane is stored in the natural gas grid. Thus, excess wind energy, via hydrogen generation, is used to eliminate CO2 in biogas and produce methane, for clean carbon-based energy. The concept has been proven at the Lemvig biogas plant, the largest in Denmark.

Slide 21- Methane gas storage for renewable energy.
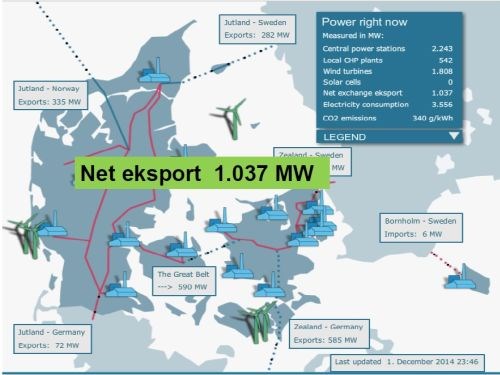
Slide 22 - Power profile in Denmark.
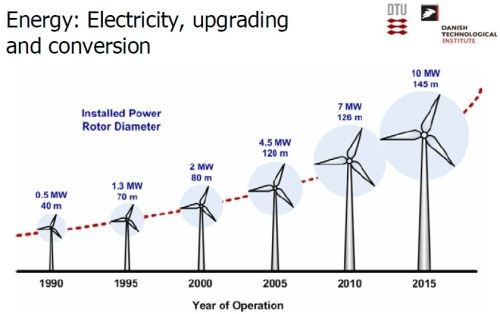
Slide 23 - Growth in individual wind turbine power capacity (1990-2015).
Hydrogen generation - electrode development
The key to the generation of hydrogen from wind energy (Slide 24), lay in the development of the catalytic electrode. Reaching back nearly 90 years, Dr. Møller found a 1927 patent (US Patent 1,628,190, Method of Producing Finely Divided Nickel), which provided the basis for the hydrogen generation (Slide 25). Finely-divided nickel, with very high surface area, was found to be extremely catalytic for hydrogenation processes. The preparation involves the mixing of nickel and aluminum, heating to form an alloy, and then selectively dissolving the aluminum, resulting in highly porous (i.e., “finely-divided”) nickel.
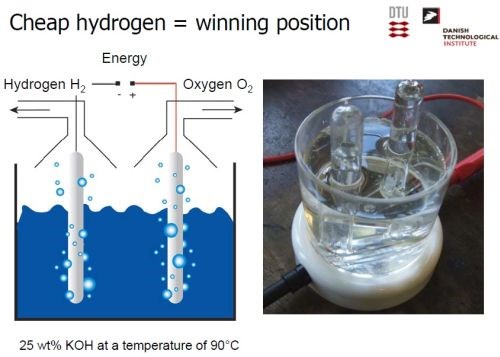
Slide 24 - Innovation in hydrogen generation.

Slide 25 - U.S. Patent for producing finely-divided nickel.
The desired electrode was produced by a combination of physical vapor deposition and electrodeposition. Nickel was electrodeposited, followed by a PVD coating of aluminum (Slide 26). Slide 27 shows the nickel operation on a pilot scale, while Slide 28 shows the PVD operation. Alloying the layers at about 620°C produces a multitude of Ni-Al intermetallics, as in the phase diagram of Slide 29. The annealing process yields a distribution of Ni-Al phases, with Ni2Al3, constituting the primary intermetallic in the bulk of the deposit near the surface (Slide 30). Finally, the aluminum was selectively leached from the Ni2Al3, resulting in an extremely porous nickel, ideal for electrocatalysis (Slides 31-32, by optical and electron microscopy, respectively)). Recent developments have improved the electrodes even further and a new solution based on electrodeposition only is being explored (Slide 33).
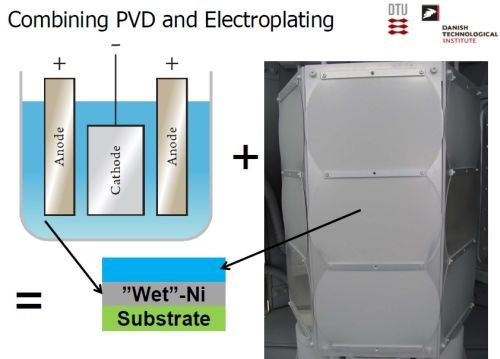
Slide 26 - Production of catalytic electrode by PVS and electroplating.

Slide 27 - Electrodeposition of nickel.

Slide 28 - Physical vapor deposition of aluminum.
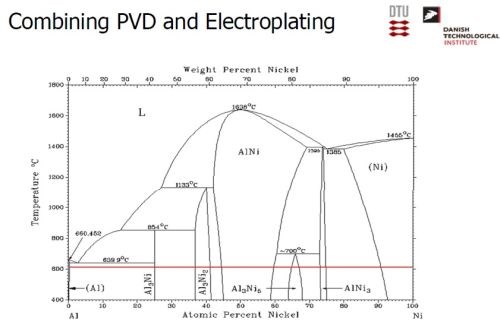
Slide 29 - Nickel-aluminum phase diagram.
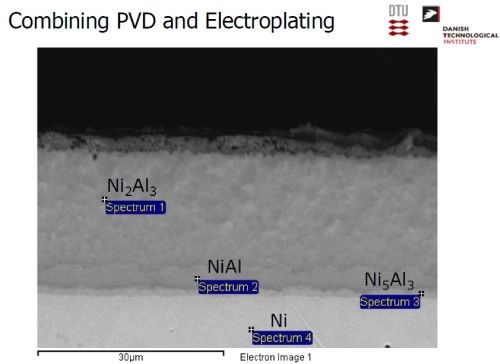
Slide 30 - Phase distribution in alloyed nickel-aluminum.
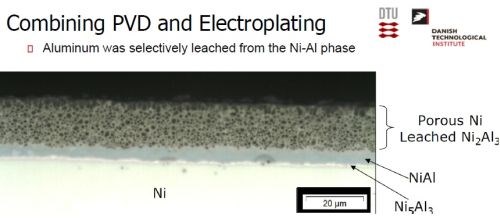
Slide 31 - Optical microscopy: Aluminum selectively leached from Ni2Al3.
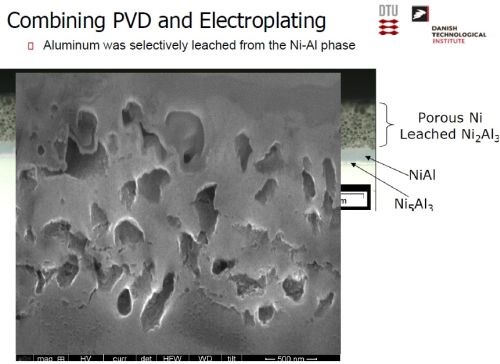
Slide 32 - SEM: Aluminum selectively leached from Ni2Al3.
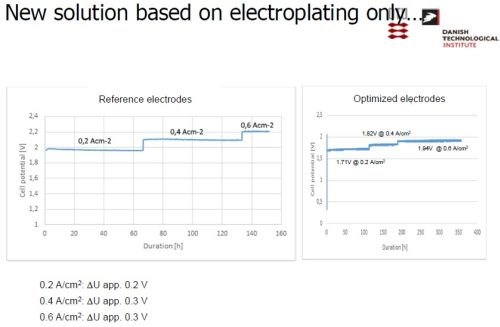
Slide 33 - Electrode manufacture via electrodeposition only.
Hydrogen generation - scale-up
Recent work has been involved in scaling up the hydrogen generation process. The nickel electrode plating operation shown in Slide 34 illustrate the scale of operation currently achievable.

Slide 34 - Electrode scale-up.
Using a bipolar principle, an array of electrodes connected in series allows production of hydrogen at significant rates (Slide 35). A full-scale unit (Slide 36) will be ready for testing in September 2018.
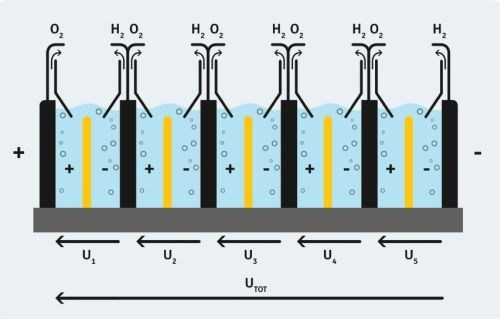
Slide 35- Schematic diagram of a full-scale hydrogen generation unit.

Slide 36- Elplatek/HydrogenPro hydrogen production unit to be tested in September 2018.
Potential for fuels
There are many aspects to the wind power-hydrogen-methane-biogas scheme that could be exploited. Among these was the potential for sustainably synthesizing fuels. If one considers the energy density of the substances considered here, it is obvious that the storage volume is critical for vehicles (Slide 37). Using an energy yardstick of 3.56 kW/hr between refueling, hydrogen would occupy 1000 L, requiring 700 atmospheres of compression to fit into a car realistically, a concept not too realistic in itself. Similarly, the methane equivalent would be 322 L, requiring 200 atmospheres of compression. However, methanol (not the ethanol used in cars in the United States) derived from methane, would occupy 0.7 L at one atmosphere. This compares with 0.4 L for petroleum at one atmosphere. Methanol would not be unrealistic.
In this way, Dr. Møller and his team envisioned the hydrogen produced by electrocatalysis from wind energy, being used to clean the biogas, thereby producing methane by reacting with the CO2 in the biogas. The methane could then be converted to methanol by dry/wet reforming (Slide 38). He calculated that the energy content in the original biogas could be increased by 50% with this system (Slide 39).
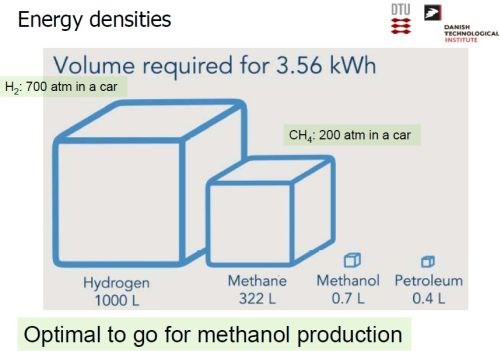
Slide 37 - Comparison of potential sustainable fuels.

Slide 38 - Modularized fuel factories.
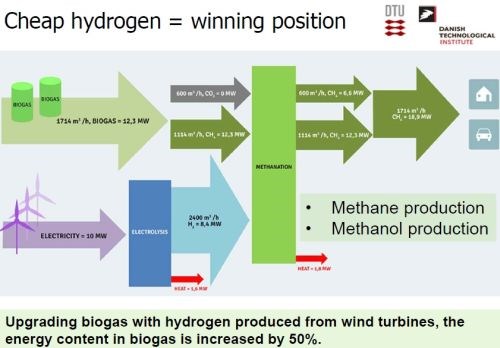
Slide 39 - Energy enhancement of biogas to produce methane and methanol.
Electrocatalytic cleaning of biogas
Given its origins, i.e., farm and industrial waste, biogas is not pure in any sense. Although it consists of 65% methane and 35% CO2, there are significant impurities (Slide 40). Of significant note is sulfur, in the form of 2000 ppm of hydrogen sulfide and, to a lesser extent, 50 ppm of methanethiol. To be useful for further catalytic conversion, the sulfur must be removed. In conventional cleaning of biogas, there are still waste considerations (Slide 41). Although the H2S is effectively reduced from 2000 ppm to about 10 ppb, the sulfur is tied up as a metallic sulfide, which must be hauled away.
An electrocatalytic process was developed by Dr. Møller and his colleagues, which converts the sulfide in the biogas to pure sulfur, which can be used commercially (Slides 42-43). There is no resultant waste. Instead, the sulfur impurity can be put to use.
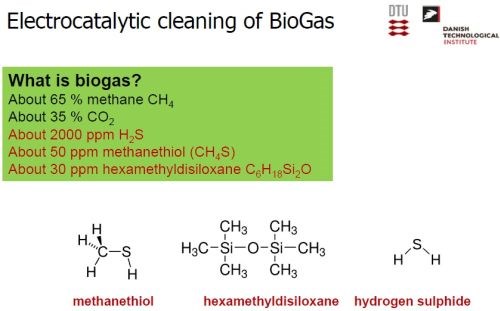
Slide 40 - Composition of biogas.

Slide 41 - Waste considerations in conventional cleaning of biogas.

Slide 42 - Unit for electrocatalytic cleaning of biogas.
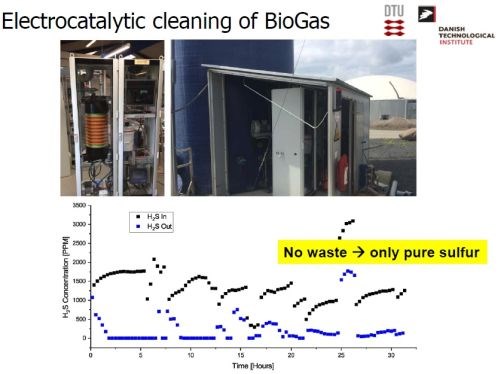
Slide 43- Impact of electrocatalytic cleaning of biogas.
Production of methane and methanol
As noted earlier (Slide 39), the next phase of this renewable energy system is the conversion of CO2 to methane. The process uses the Sabatier reaction. Once again using the hydrogen from the wind-driven electrocatalysis, reaction with the carbon dioxide in the biogas produces methane and water. This requires a nickel catalyst (Slide 31) at a reaction temperature of 300 to 400°C (Slide 44). Such reactors, shown in Slides 45-46, are available on a commercial scale. Ultimately, the methane is converted to methanol fuel (Slide 47).
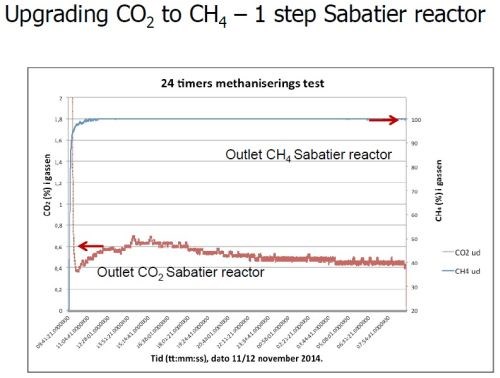
Slide 44 - Upgrading CO2 to methane - Sabatier reactor.

Slide 45 - Upgrading CO2 to methane - Pilot reactor.

Slide 46 - Upgrading CO2 to methane - Commercial reactor.
In summary, the overall strategy for this renewable energy concept is shown in Slide 47. The biogas derived from organic waste, once cleaned for sulfur (in pure form), yields CO2 and CH4. The hydrogen derived from wind-driven electrolysis is then combined to synthesize methanol.
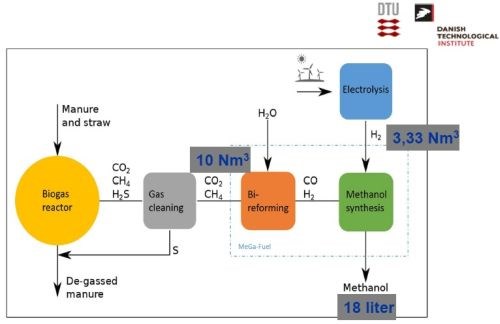
Slide 47 - Overall concept of extracting sustainable energy from wind power and biogas.
Methanol as a fuel
In North America, attention on alternative automotive fuels has been focused on ethanol. However, methanol has often been used in automotive racing, and is the focus in other areas of the world (Slide 48). Indeed, methanol is widely used in China (Slide 49). It is entirely reasonable to expect that wider use of this fuel offers a sustainable alternative fuel for the future.

Slide 48 - Commercially-available methanol fuel.
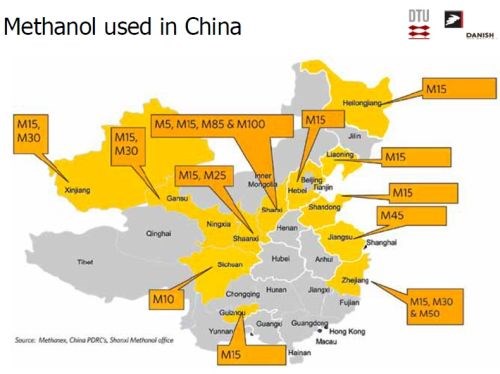
Slide 49 - Distribution of methanol fuel in China.
Dr. Møller’s work in this area has been rather significant. Indeed, it has gotten play in the newspapers in Denmark (Slide 50). In English, (via a loose computer translation), the headline reads, “How to get a jumbo jet to fly on wood alcohol.”

Slide 50 - Reaction to Dr. Møller’s work in Denmark.
Self-cleaning paints
Another avenue of surface research has led Dr. Møller to look at self-cleaning paints. Such a paint would be hydrophobic and oleophobic, repelling both water and oils, respectively. The paint would continue to maintain this property even after scratching or scuffing. It would keep pollen, insects, stains, etc., from sticking to the surface.
It was found that titanium dioxide is widely used as a pigment in paints, cosmetics, etc. Anatase, a tetragonal form of TiO2 - the others being rutile, the most common form, and brookite, an orthorhombic form - possesses photocatalytic surface properties. Photoactive titania in the form of anatase was seen as a means of forming a self-cleaning surface, in addition to several other applications (Slide 51).
Slide 52 shows the performance of a normal paint, and a self-cleaning one, containing anatase, before and after exposure to 28 hr of UV. As can be seen, the blue stain on normal paint is unaffected, while it disappears from the surface of the self-cleaning paint.

Slide 51 - Basis for titania in self-cleaning paints.
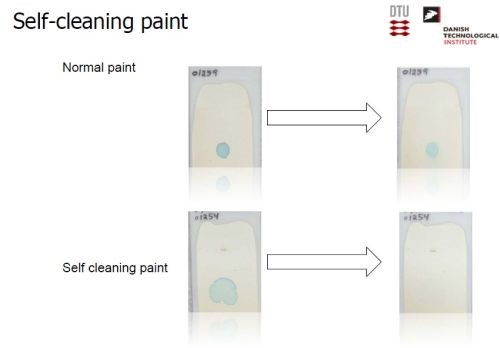
Slide 52 - Laboratory performance of self-cleaning paint.
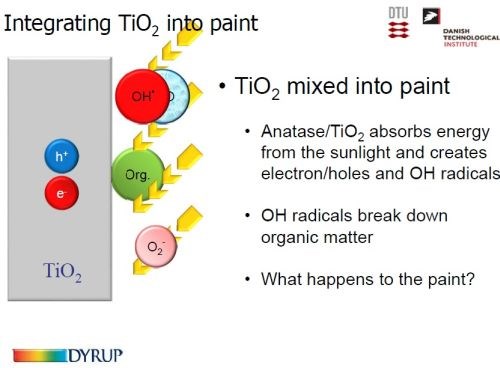
Slide 53 - Integrating titania into paint: (1) the concept.
When anatase is mixed in with the paint, its photoactive surface absorbs the energy from the sunlight, creating electron holes and OH- radicals (Slide 53), which in turn break down the organic matter forming CO2 and H2O.
When nano-sized TiO2 particles are mixed into the paint (Slide 54), the contact area between the organic binder material and the particles is extremely high. At the surface, the binder is affected by the photocatalytic reaction, resulting in a massive release of TiO2 from the surface. Thus, an organic binder cannot be used, as it leads to poor durability, and the usual unsightly chalking occurs.
The solution to this problem was the use of TiO2-coated glass beads (Slide 55). The beads are deeply imbedded into the film and do not fall out. There is considerably less direct contact between the TiO2 and the binder. As a result, minimal organic binder is affected by the photocatalysis, and a long lasting, self-cleaning film is obtained.
Dr. Møller has worked with a commercial paint manufacturer to perfect the incorporation of TiO2 in the matrix via a patented process (Slide 56-57). An atomic layer deposition (ALD) process in a specially constructed reactor converts titanium chloride (TiCl4) to TiO2 at exceedingly small thicknesses on the glass bead surface (20-30 nm). The result is a self-cleaning paint of high durability.
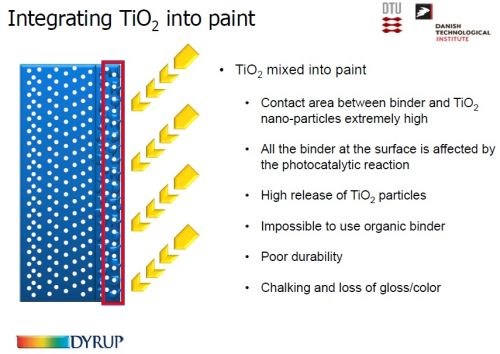
Slide 54 - Integrating titania into paint: (2) by mixing.
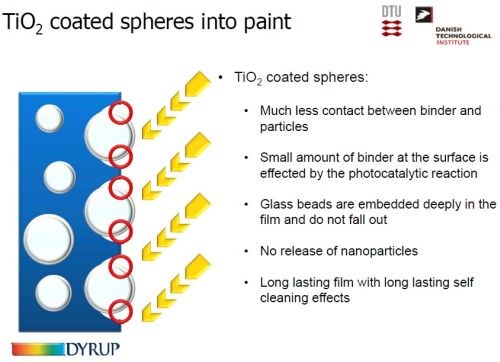
Slide 55 - Integrating titania into paint: (3) using TiO2-coated spheres.
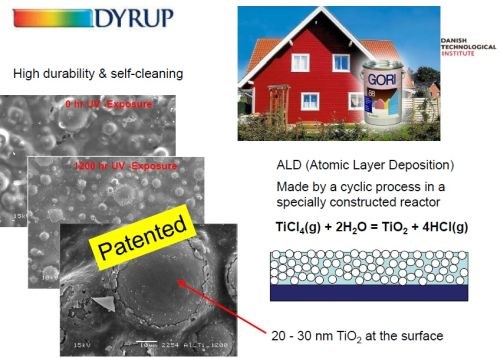
Slide 56 - Integrating titania into paint: (4) commercially available.

Slide 57 - Integrating titania into paint: (5) patented process.
Antibacterial Ag-Cu surfaces
Another part of Dr. Møller’s work involves the development of antibacterial surfaces. Consider the common door handle in a public building, e.g., a hospital, with hundreds of hands, containing oils and bacteria of unknown origin, ready to raise a public health issue at a moment’s notice. The solution was a silver-copper alloy finish known to possess anti-bacterial properties (Slide 58). And again, news of this work became well known in the local papers (Slide 59). In English, (via another loose computer translation), the headline reads, “Door handle gives bacteria a fight to the finish.”

Slide 58 - Morphology of antibacterial Ag-Cu surface.

Slide 59 - News article on antibacterial-coated door handles.
The mechanism whereby the antibacterial action takes place depends on the alloy content and the presence of a porous microstructure capable of retaining moisture. When a bacterium makes contact with the Ag-Cu deposit, oxidation of the copper and the corresponding reduction of silver produces copper ions and hydroxyl ions on the surface, killing the organism (Slide 60).
Testing involved the exposure of Ag-Cu coated test slides to a number of very common bacteria species to the contact killing mechanism (Slide 61). Dry conditions were specified, and re-inoculations of the bacteria on the test surface were undertaken every three hours for up to 21 hours, i.e., multiple times. Success was defined as a minimum of 90% reduction in numbers of bacteria at all recovery times. The results were compared with tests on an uncoated 316 stainless steel reference surface. The results shown in Slide 62 indicate the effectiveness of the Ag-Cu surface in eradicating all types of bacteria tested with a killing rate of six to eight orders of magnitude. Slide 63 shows visual evidence, with the color changing from green (live bacteria) to red (dead bacteria) indicating extermination of the bacteria after 25 min. exposure to the Ag-Cu coating.
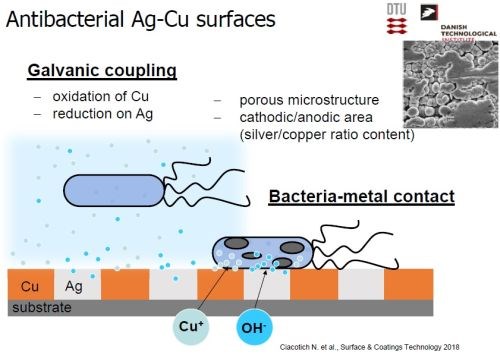
Slide 60 - Antibacterial mechanism.

Slide 61 - Antibacterial test protocol.
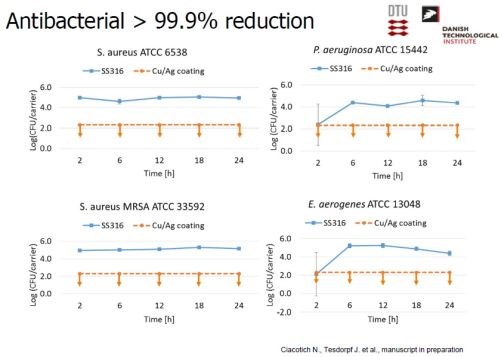
Slide 62 - Antibacterial test results (1).
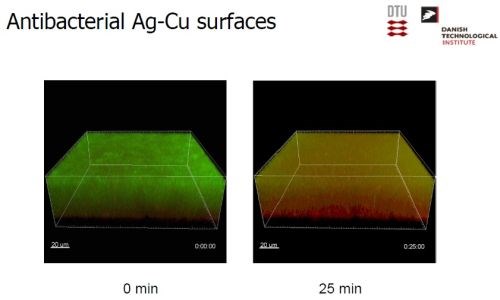
Slide 63 - Antibacterial test results (2).
Summary
To sum up (Slide 64), Dr. Per Møller’s work has covered a multitude of applications where surface technology is critical to success. In concert with his longtime colleague, Dr. Lars Pleth Nielsen, he has documented this work through patents, papers and renowned books. The scope of his work goes beyond the more common applications found in deposition. The sustainable energy scheme described on these pages, using the principles of electrocatalysis, promises to leverage the maximum amount of energy from wind power and biogas, the latter a resource that would otherwise be considered as pure waste in other times. The work with self-cleaning paints and antibacterial surfaces are other examples of novel applications requiring an understanding of electrochemistry and surface technology in real applications outside laboratory conditions which is indeed one of Dr. Møller’s many skills.

Slide 64 - Summary
About the lecturers

Professor Per Møller has a Ph.D. in surface technology from the Technical University of Denmark (DTU), in Lyngby, Denmark. During the past 30 years, he has been engaged in contract research with industry covering nearly all aspects from micro-plating, under cleanroom conditions, to the design and implementation of industrial-scale electroplating lines. He is author or co-author of more than 135 scientific papers and holds more than 25 patents in the field of surface technology and electrochemistry. Currently, he is Professor in corrosion and surface technology at the MEK-DTU Section for Materials and Surface Engineering.

Dr. Lars Pleth Nielsen has a Ph.D. in Surface Science from Aarhus University (Denmark) and a managerial degree in Organization Management and Innovation from Copenhagen Business School. He has been employed as a Research Scientist at Haldor Topsøe and in the Photonic Group at NKT Research and Innovation A/S. Currently, he leads the Tribology Center at the Danish Technological Institute.
Related Content
Portfolio of Energy-Curable Coatings Launched
PPG's DuraNEXT portfolio includes EB and UV curable coatings, offering rapid, energy-efficient solutions for metal coil coaters.
Read MorePractical Environmental Management Reduces Costs, Refines Quality
By focusing on effluent treatment and efficient tin recovery, this Indian surface treatment plant meets stringent environmental standards and sustainable high-quality production.
Read MoreEnergy Efficient SprayWand Pretreatment System
P-450 Energy Efficient SprayWand Pretreatment System Source: PEM PEM Inc., a leader in providing innovative batch and automated finishing equipment to OEMs, fabricators and job shops, will display its SprayWand, FinFlow and SprayLean brands.
Read MoreAkzoNobel Opens World-First Testing Facility for Wind Turbine Blades
AkzoNobel's state-of-the-art wind turbine blade testing site simulates extreme weather and runs high-speed tests, driving innovation in protective coatings.
Read MoreRead Next
Delivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read More












.jpg;maxWidth=300;quality=90)








