Low Temperature Curing of Hydrogen Silsesquioxane Surface Coatings for Corrosion Protection of Aluminum
This paper discusses vitreous enameling of hydrogen silsesquioxane (HSQ).
by
Felix Lampert*, Annemette Hindhede Jensen** and Per Møller† *
*Technical University of Denmark (DTU), Lyngby, Denmark
**SiOx ApS, Espergærde, Denmark
Editor’s Note: This paper is a peer-reviewed and edited version of a paper as part of a presentation delivered at NASF SUR/FIN 2015 in Rosemont, Illinois on June 9, 2015. A printable PDF version is available by clicking HERE.
ABSTRACT
Hydrogen Silsesquioxane (HSQ) has shown to be a promising precursor for corrosion protective glass coatings for metallic substrates due to the excellent barrier properties of the films, especially in the application of protective coatings for aluminum in the automotive industry where high chemical stability in alkaline environments is required. The coatings have been successfully applied to stainless steel substrates. However the traditional thermal curing of HSQ involves heating to elevated temperatures, which are beyond those applicable for most industrial applications of aluminum. In this study low temperature processes are tested and evaluated as possible alternatives to the traditional high temperature cure. Thin HSQ films are deposited on silicon wafers to model the degree of curing induced by the low temperature methods in comparison to thermal curing. Furthermore, the coatings are applied on aluminum substrates to evaluate the adhesion and corrosion resistance of the films.
Keywords: Aluminum, corrosion, electron beam curing, hydrogen silsesquioxane, HSQ, vitreous enameling
Introduction
Vitreous enameling is a well-known coating technology for the corrosion protection of metals due to the outstanding barrier properties of silica glass.1 The E-pH diagram shown in Fig. 1(a) displays silica as a largely inert material in the pH range from 0 to 11-12 in aqueous media. A transfer of these inert properties in form of a coating is particularly interesting for metals such as aluminum, where a passive region is only observed in the range between approximately pH 5-8 (Fig. 1(b)). The narrow stability range limits the industrial use of aluminum. Besides a curing temperature above the liquidus temperature of vitreous enamel close to the melting temperature of aluminum and a large coating thickness, traditional vitreous enameling often becomes problematic with respect to tolerances and flexibility of the coatings.
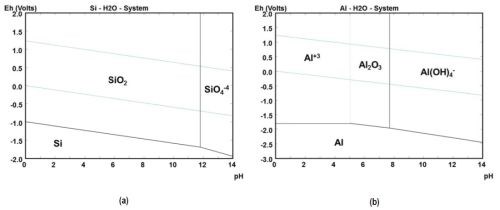
Figure 1 - E-pH diagrams at 25°C, pressure 1 bar and molarity 1E-6 for (a) silicon dioxide and (b) aluminum. Diagrams were calculated with HSC Chemistry 5.11 software.
Advances in hydrogen silsesquioxane (HSQ) technology2 have shown the feasibility of overcoming these issues by the deposition of sub-micrometer thick silica coatings at comparably low temperatures. The coatings provide excellent corrosion protection, while maintaining a good resilience against brittle cracking. In this perspective, the technology enables the application of thin protective glass coatings in applications where a high degree of corrosion resistance, combined with high geometrical needs, is required. Accordingly, the technology shows promising results, e.g., in the field of heat exchangers, due to increasing corrosion resistance and durability, while maintaining good thermal conductivity,2 or in decorative applications, where resistance against atmospheric corrosion can be increased without sacrificing the optical appearance of the product. Furthermore, the material shows excellent surface smoothing abilities3-5 and recently the application of HSQ coatings in polymer molding tools, where high precision, low surface roughness and high durability are required, has been reported.6 Besides the improvement in corrosion protection, the coating is expected to fulfill special hygienic needs, due to the good cleanability of the smooth surface, or decrease the adhesion of biological films in fouling reduction in offshore applications. Our current research has focused on a variety of materials, such as ferritic, austenitic and martensitic steels, aluminum, copper, brass, nickel and silver. However, an even broader range of possible substrates is expected in our studies.
In state of the art processes, HSQ is applied in solution and subsequently heat treated to cross link the cage-like oligomers to a silica-like, networked inorganic polymer,7 as shown in Fig. 2. However, the efficiency of the transformation is strongly dependent on the curing temperature8 and until now curing temperatures above 400°C are necessary to sufficiently protect substrates from corrosion.2 Consequently the current process is widely applicable on substrates such as steels, which do not undergo significant softening or loss of mechanical properties at the required temperature. However it is not suitable for the industrial application of thin glass coatings on engineering components made from aluminum, which undergo extensive softening at these elevated curing temperatures.
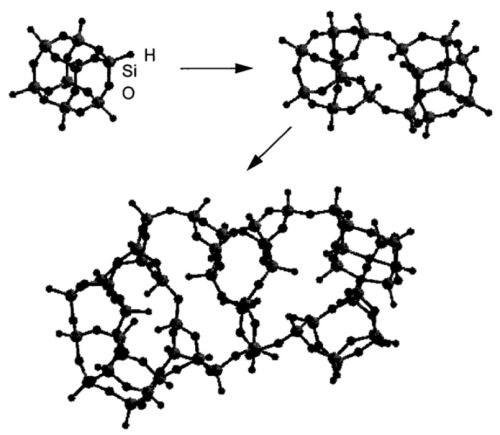
Figure 2 - Conceptual illustration of the HSQ cure process from cage-like structure to network structure; illustration by Bremmer, et al.7
Besides thermal curing, electron beam exposure has shown to induce similar cross-linking reactions in HSQ.9-11 In this perspective it has been shown that electron beam curing can lead to the same degree of cross-linking like thermal curing at 400°C in a protective atmosphere10 and it has been suggested that a further increase in electron beam dose might lead to even higher degrees of curing.11 However, studies with electron beam doses exceeding 5 MGy†† are absent and the effect of irradiation in elevated doses on the curing behavior of HSQ needs to be assessed.
In this work, the effect of electron beam exposure up to 12 MGy (Megagray) on HSQ films was assessed with respect to the induced networking and compared to thermal curing at 450°C in ambient atmosphere. Both, thermally and electron beam cured HSQ films will be applied to aluminum substrates and the corrosion properties of the films will be assessed.
Experimental methods
Si-wafers (Topsil FZ wafer, 675 µm, front side polished, backside etched) and Al 1050 sheets (1 mm thickness, roughness Ra = 0.066 µm / Ra = 0.131 µm in dipping / perpendicular to dipping direction; roughness measured on an optical profilometer, Hommel tester with cone diameter 2 mm) were dip coated in Dow Corning FOX® 25 flowable oxide on a Thorlabs LTS300 linear translation stage. Dipping with 0.25 mm/sec for silicon wafers resulted in a film thickness of 0.17 µm on the silicon wafers (after solvent evaporation) and dipping with 1 mm/sec resulted in a film thickness of up to approximately 0.7 µm on the aluminum specimens (after curing).
Prior to coating, the silicon wafers were cleaned in organic solvents (ultrasonic cleaning in ethanol and subsequent cleaning in acetone) and the aluminum specimens were degreased, oxide-stripped in NaOH (20 g/L) for 45 sec, desmutted in HNO3 (30 vol%) + NaF (10 g/L) for 45 sec and cleaned in acetone.
After coating, the solvent was evaporated by a soft bake in an air furnace at 160°C and the specimens were cured by the following methods: Reference samples were annealed at 450°C for 2 hr in ambient air and subsequently air cooled. Electron beam curing was performed on a Comet EBLab low energy accelerator in ambient atmosphere with an acceleration voltage of 100 kV and a beam current of 5.281 mA. The resulting irradiation dose was 38.6 kGy/cycle (determined by Risø High Dose Reference Laboratory using GEX DoseStix dosimeters) and higher doses were achieved by application of multiple cycles. The corrosion resistance on the aluminum substrates was assessed on uncoated (Specimen U), thermally cured (Specimen T) and electron beam cured (Specimen E) specimens. The specimen designations are summarized in Table 1.
Table 1 - Specimen designations for the aluminum specimens.

The specimens were characterized by stereo microscopy and scanning electron microscopy (SEM) / focused ion beam (FIB) cross-sectioning on a Helios Nanolab 600 FEG SEM. EDS analysis was carried out with an Oxford X-Max 50 mm2 by the Danish Technological Institute (DTI). The film thicknesses (t) and refractive indices (n) of the films on silicon wafers were determined by ellipsometry on a VASE ellipsometer and the film thicknesses on aluminum specimens were estimated from FIB cross-sections. The degree of cross-linking was assessed by Fourier transform infrared (FTIR) spectroscopy, measured on a Thermo Scientific Nicolet iN10 MX in transmittance mode. The data presented are digitally modified by baseline subtraction and removal of atmospheric influence. To exclude effects from thickness inhomogeneities due to the dip coating process all point-measurements were taken in the middle of the specimens and 15 mm from the drop-off edge.
Linear polarization measurements were performed in a flat cell with 100 mL Sodium acetate 0.5M buffer solution at pH 6 and an Ag/AgCl reference electrode, without stirring of the electrolyte. The scan rate for the experiments was 1 mV/sec on an area of 0.95 cm2. Prior to the recording of the curves, the open circuit potential (OCP) was determined for 1800 sec. All scans were performed on an ACM Instruments GillAC potentiostat.
Salt spray testing was carried out for three weeks at 35°C. The sodium chloride solution was prepared with 50 g/L NaCl in deionized water according to ISO 9227:2012(E).12
Results and discussion
Curing on Si wafers
As shown in Fig. 3, the FTIR spectra of the soft baked HSQ film show absorption peaks at 1130 and 1070 cm-1, referring to Si-O bonds in cage and network structure respectively,13 and an absorption at 2285 cm-1, referring to Si-H stretching.14 Si-O cage absorption clearly dominates the Si-O network absorption, leading to the conclusion that the measured film largely consists of non-crosslinked HSQ cage molecules. Absorption in the region from 3000 to 3700 cm-1, originating from hydroxyl stretching,15 is not visible from the spectrum. Thermal annealing at 450°C in air leads to a transformation from cage to network structure, accompanied by a decrease in Si-H. In addition to the peaks from Si-O and Si-H, a broad hydroxyl stretching peak between 3000 and 3700 cm-1 can be observed. A similar peak has been observed by Bremmer, et al.,7 who suggested that HSQ can convert to silica via oxidation of Si-H via an intermediary silanol phase, which can subsequently induce moisture absorption.
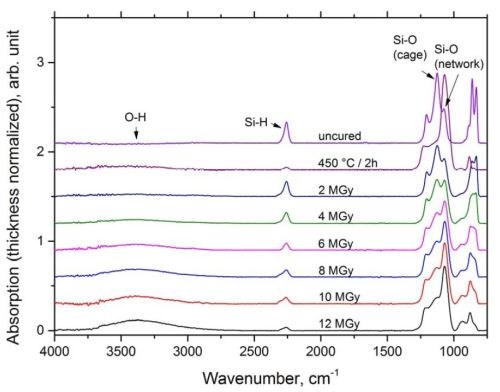
Figure 3 - Thickness normalized absorption in FTIR for soft-baked, thermally cured and e-beam cured with irradiation doses in the range from 2 to 12 MGy.
Similar to thermal curing, electron beam curing influences the bonding structure of the film. With increasing irradiation doses, the peak ratios between Si-O cage and Si-O network shift toward higher Si-O network ratios, while the absorption from Si-H continuously decreases. From the experiments it becomes obvious, that the effect of electron beam irradiation on the cross-linking of the Si-O network in the applied dose ranges is less significant than from the thermally cured reference specimen. Despite the previously described peaks the FTIR spectra from the electron beam cured specimens show a broad hydroxyl absorption between 3000 and 3700 cm-1. To the knowledge of the authors, a broad hydroxyl absorption in the region from 3000 to 3700 cm-1 has not yet been reported in literature for electron beam cured HSQ films. In contrast to previous studies,9-11,16 we report electron beam curing in air without a protective atmosphere and therefore the films have access to oxygen as well as moisture. As a consequence, a possible mechanism for hydroxyl absorption is suggested based on the electron beam curing model proposed by Namatsu, et al.16 According to the group, HSQ is crosslinked via intermediate silanol formation, which can in turn induce moisture absorption, similarly to thermal curing in oxidative atmosphere.
Direct comparison between thermally and electron beam cured films points out that the curing induced by electron beam curing is less efficient than thermal curing at 450°C in air, while a higher amount of silanol/water is present in the electron beam cured films.
Corrosion tests on coated aluminum
From the polarization curves shown in Fig. 4, it becomes evident that the rate of corrosion is generally higher for specimen U compared to specimens T and E. Furthermore, the corrosion potential increases from -321 mVSHE for specimen U to -220 mVSHE for specimen T and -38 mVSHE for specimen E. In contrast to specimen U, specimens T and E did not show passivation of the surface, accompanied by a sudden reduction in corrosion current, followed by a limiting current. The high noise ratio on the curve of specimen T suggests a dissolution mechanism based on strong local dissolution or pitting at coating defects such as cracks. This is in accordance with investigations of the surface, showing substrate dissolution through cracks as the main corrosion process on the area stressed during linear polarization measurements on specimen T (Figs. 5(a) and (b)). Coating failure of specimen E after linear polarization measurement was not observed.
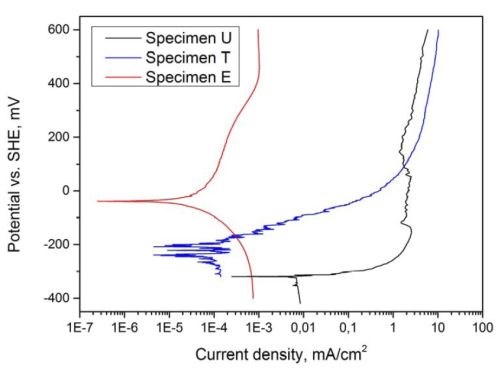
Figure 4 - Linear polarization curves of Al-specimens, for specimen designation see Table 1.
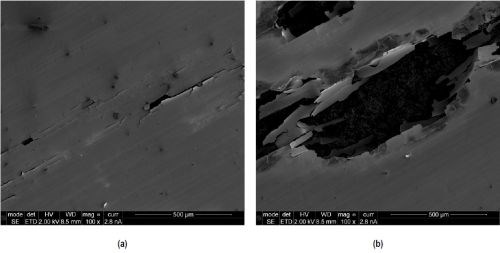
Figure 5 - SEM micrographs of coating failure of specimen T after linear polarization measurement.
Salt spray testing has shown that both thermally and electron beam cured specimens (Fig. 6(a-c)) are less affected after exposure. Specimen U, displayed in Fig. 6(a), shows uniform corrosion covering the entire surface. In contrast, specimen T exhibits a generally uncorded surface with isolated cloudy regions, while specimen E shows cloudy regions dispersed over the surface. For both specimens, the cloudy regions can be identified as spallation of the surface layer, as shown by the SEM micrograph in Fig. 7. FIB cross-sectioning (Fig. 8) shows that the cracked surface layer originates from a surface deposit on top of the silica coating that has cracked and flaked off. EDS analysis on the FIB cross sections identifies the flaky surface film as an aluminum-rich deposit and it is suggested that the surface film originates from redeposited corrosion product.
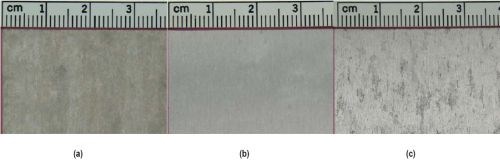
Figure 6 - Appearance after three weeks of salt spray testing: (a) Specimen U; (b) Specimen T and (c) Specimen E.
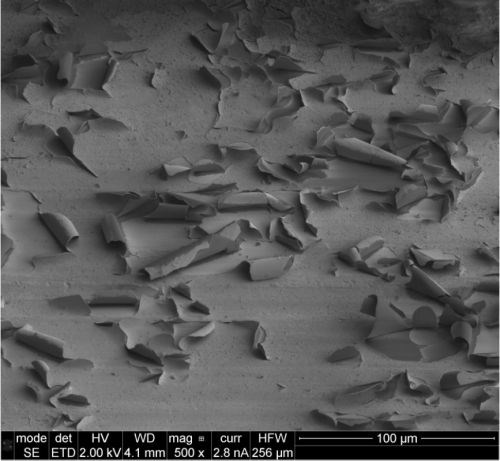
Figure 7 - SEM micrograph of coating failure on specimen E after salt spray testing. The image shows spallation of a surface deposit.
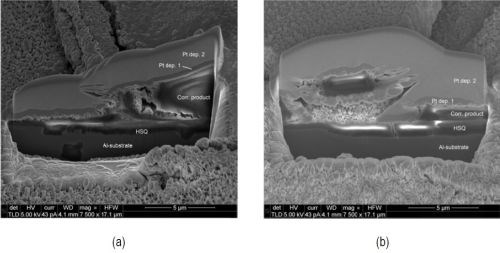
Figure 8 - FIB cross-sections of surface deposit after salt spray testing: (a) Specimen T and (b) Specimen E. The images show a good adherent HSQ layer on the surface and spallation of a surface deposit. Two layers of platinum have been deposited to protect the area locally from FIB artifacts (Pt dep. 1 and 2). The image has been corrected for specimen tilt on the cross-sections.
The better performance of specimen E in the polarization experiment suggests that electron beam cured films are, in contrast to thermally cured films, initially defect free, i.e., no anodic dissolution via pitting at coating defects can be observed. However, upon extended immersion times, the electron beam cured coatings show poorer protective behavior, leading to the more pronounced failure observed in salt spray testing. Since specimen T showed good performance despite the coating defects observed in the polarization experiment, it is suggested that the thin barrier coating decreases the cathodic area and thereby effectively slows down uniform corrosion by inhibition of the cathode reaction, even in the presence of coating defects.
Conclusion
Both electron beam irradiation and thermal curing have shown to induce cross linking of the surface film to a silica-like glass coating, which acts as a protective barrier coating and can significantly slow down corrosion of aluminum. However, the Si-O network formation induced by electron beam curing, even at elevated irradiation doses, was less complete than for thermal curing. Electron beam cured coatings resulted in seemingly better results in potentiostatic polarization studies, however showed poorer durability during salt spray testing. Based on the observations it is suggested that thin glass coatings protect metallic substrates from uniform corrosion by reducing the cathodic area and thereby inhibiting the cathode reaction, which has also shown to be efficient in the presence of coating defects. Generally, thin silica films have shown to minimize the corrosion rate of aluminum substrates and have proven to be promising coatings for metallic materials.
References
1. P. Møller & L.P. Nielsen, Advanced Surface Technology, Vol. 2, ISBN: 978-87-92765-239, 2013; pp. 645-654.
2. H. Pranov, U.S. Patent Application 2014/0154441 A1 (2014).
3. K. Mohaghegh, et al., Proc. 14th euspen International Conference (2014).
4. K. Mohaghegh, et al., Proc. 13th euspen International Conference (2013).
5. H. Pranov, International Patent WO 2013/083129 A1 (2013).
6. J. Cech, et al., Appl. Surf. Sci., 280, 424-430 (2013).
7. J.N. Bremmer, et al., Proc. Mater. Res. Soc. Symposium, 476, 37-44 (1997).
8. Y.K. Siew, et al., J. Electrochem. Soc., 147, 335-339 (2000).
9. D.L Olynick, et al., J. Vac. Sci. Technol. B, 28, 581-587 (2010).
10. T. Nakamura, et al., Jpn. J. Appl. Phys., 40, 6187 (2001).
11. S. Choi, et al., J. Vac. Sci. Technol. B, 26, 1654-1659 (2008).
12. ISO 9227:2012, “Corrosion tests in artificial atmospheres - Salt spray tests” (2012).
13. W.C. Liu, et al., J. Non. Cryst. Solids, 311, 233-240 (2002).
14. C.L. Frye and W.T. Collins, J. Am. Chem. Soc., 92 (19), 5586-5588 (1970).
15. G.W. Griffith, Ind. Eng. Chem. Prod. Res. Dev., 23, 590-593 (1984).
16. H. Namatsu, et al., J. Vac. Sci. Technol. B, 16, 69-76 (1998).
Footnotes:
†Corresponding author:
Dr. Per Møller
Technical University of Denmark
Produktionstorvet, BuildinHi-Lite this text then type your caption here.g 425,
2800 Kgs. Lyngby, Denmark
E-mail: pm@epc-info.dk
††1.0 Gy: Absorption of 1.0 Joule of ionizing radiation per kg of matter.
About the authors



Related Content
NASF/AESF Foundation Research Project #121: Development of a Sustainability Metrics System and a Technical Solution Method for Sustainable Metal Finishing - 15th Quarterly Report
This NASF-AESF Foundation research project report covers the twelfth quarter of project work (October-December 2023) at Wayne State University in Detroit. In this period, our main effort focused on the development of a set of Digital Twins (DTs) using the Physics-Informed Neural Network (PINN) technology with application on parts rinsing simulation.
Read MoreSUR/FIN 2023 Registration Is Now Open
The National Association for Surface Finishing SUR/FIN 2023 surface finishing industry trade show will take place June 6-8, 2023 in Cleveland, Ohio.
Read MoreHexavalent-Chromium-Free Aluminum Sacrificial Paint Validation
Hexavalent chromium is a known carcinogen, repro-toxin and mutagen. Its elimination is of high importance to the aerospace industry, which has struggled to find high performing alternatives. Legacy aluminum sacrificial paints have traditionally utilized hexavalent chromium to prevent corrosion and coatings which are equal to or better than have been difficult. This second of two papers discusses the hexavalent-chromium-free process from the user point-of-view in terms of the process validation work by Rolls Royce Corporation.
Read MoreSUR/FIN 2023: Capsules from the Technical Sessions I: Emerging Technologies
SUR/FIN 2023 in Cleveland this past June was a resounding success. Due to the efforts of the Technical Activities Committee, ably led by Bill Nebiolo this year, an outstanding program of technical presentations was offered. What follows are summaries of selected presentations from the Emerging Technologies sessions. Additional coverage will be provided in this space in the coming months. The full report can be accessed and printed at short.pfonline.com/NASF23Aug1.
Read MoreRead Next
Delivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read More











.jpg;maxWidth=300;quality=90)














