Palladium-Free Direct-Plate Technology for Plating on Plastics
Plating on plastics traditionally has been accomplished using tin/palladium colloidal-type activation systems with electroless chemistries. Although well understood, these processes can be costly and require extended process lines with high water consumption...
A method to electroplate directly onto non-conductive plastics has been developed. The technology does not use precious metals to activate or electroless chemistries to produce the first conductive metallic layer. Instead, the process allows electrolytic nickel to plate directly onto the plastic substrate. This direct-plate technology decreases the number of process steps, significantly reduces water consumption, eliminates chelates and improves productivity.
A direct metallization process eliminates the need to use electroless chemistries and shortens the pretreatment time by reducing the number of processing steps. This direct-plate technology employs a mild etching process that ensures a high degree of adhesion. It has been used in production in several pilot plants since 2001.
Increasingly stringent environmental conditions, as well as substantial cost reductions called for by the automobile industry have forced the electroplating industry to look for techniques such as direct metallization processes.
To reap the benefits of direct-plate systems, certain processing considerations must be well understood. The etching process is an important factor and is responsible for the degree of adhesion on both ABS and ABS/PC resins. The etching process is also responsible for the actual ability to metallize such plastics. The best possible degree of adhesion and "metallization" for the direct-plate process is achieved using a mild etch.
Mild Etch
Figure 1 shows an ABS surface that has been etched in a conventional colloidal ABS process chromic-sulfuric acid etch. The rough, heavily attacked surface required by the classic type of colloidal activation processes is depicted here. This aggressive etch is necessary for proper adhesion in a colloidal process. As a result of such strong roughening, the surface of the molded part may be destroyed. The result could impact adhesion and degrade the surface quality.
Figure 2 shows a surface that has been etched under mild conditions in the direct-plate process. This slightly roughened plastic surface with individual cavities is well suited for the "buttonhole" adhesion mechanism between the surface of the substrate and the metal deposit. This is very important as it provides a high degree of adhesion while maintaining the "Class A" as-molded parts’ surfaces.
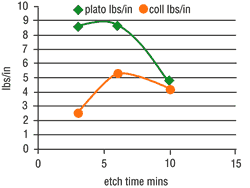
Etching parameters directly influence surface chemistry. The mild etch enhances the formation of polar functional groups (-OH, -C=O, -COOH) on the plastic surface, where the percentage of hydroxyl groups (-OH) are found to be particularly high. These make a chemical link with the positively charged activator molecules possible. In the following conducting solution, the active metal is "wetted" to the plastic substrate via a bridge of sulfur as a result of a kind of vulcanization.These two reactions achieve an additional chemical adhesion. The results of adhesion investigation are shown in Figure 3.
Wide Scope of Application
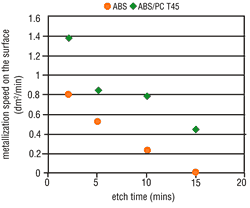
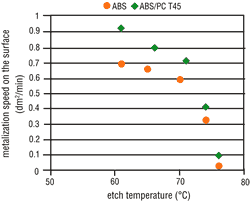
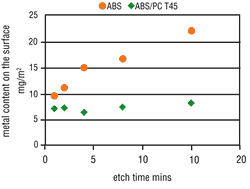
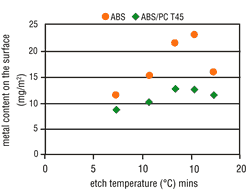
Tests clearly have shown that the possibility of metallizing a particular surface is decisively dependent upon the applicable etching conditions. The metal activation on the ABS surface increases in line with the etch time (Figure 4) and the etch temperature (Figure 5). Thus, by raising the etching parameters we get a corresponding increase in micro-structuring, the result is an increase in the area size. ABS/PC surfaces do not undergo such a critical
change.
From experience, deposit coverage is achieved more quickly by increasing the amount of surface metallic activation. This property also is seen with the use of direct metallization-based palladium activators. Consequently, the metallization speed on ABS should increase with higher etching parameters, but on ABS/PC this is less significant. As opposed to expectations, the metallization speed on both types of material becomes slower when the etching parameters are increased. At certain etching times and temperatures, complete inhibition may occur (Figures 6 and 7).
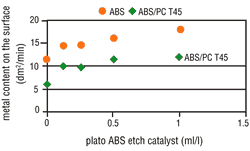
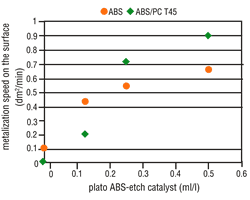
Under increased etching conditions, the effective plastic area per area unit increases. A reduction in area growth is, therefore, understandable. Complete metallization inhibition cannot be, however, similarly explained. It is quite possible that the etching parameters will affect the percentage and composition of the functional groups on the plastic surface. The presence of hydroxyl groups (-OH) with mild etches is far higher than after strong etching. However, the activator contains both "active" and "inactive" metallic compounds that are deposited on the plastic surface. Therefore, it seems that hydroxyl groups enhance the deposition of "active" molecules on plastic surfaces. Based on this theory, a catalyst was used that further reduced the attack by etching, but also simultaneously enhanced the formation of increased hydroxyl groups on the plastic surface. By adding this etch catalyst, the number of "active" molecules increased and at the same time the metallization speed also increased (Figure 7 and 8). This step considerably widens the scope of application for the direct-plate process.
Reduction of Process Steps
Activation is carried out following a mild etch and subsequent rinsing in a complex metal amine solution. With the direct-plate process there is no need for chromium reduction. The quality of the activator, i.e., its operational life and its "activity," depend on the conditions applicable during production. Drag-out and work volume reduce the quality of the activator, but this is easily managed by replenishment. Temperatures higher than 30°C can have a negative impact on the activator. The immersion time in the activator has no effect on the metallization process. One must only ensure that the components are completely "wetted" after four minutes.
After activation, parts are given an alkaline rinse. Hydrolysis washes off loosely adherent complex metals as metallic hydroxides from the rack insulation. Immersion in the conditioner should be less than two minutes, since complex metals would be chemically removed from the surface of the production parts as a result of hydrolysis. The final sulfide conducting solution produces a grey, firmly adhering film that consists of metal and polysulfides. During the reaction that occurs between the complex metal and the sulfurous solution, further reactions take place on the highly active plastic surface. Here again, the immersion time has no effect on the metallization; one minute is sufficient
For the first electroplating bath, only electrolytes with potential lower than the reduction potential of the sulfide activator deposit are used. Typically, nickel is used since the potential from acid copper electrolytes is too electropositive. Tests show that the sulfamate-nickel has excellent metallizing characteristics. The voltage must start at two to three volts and increased step-by-step. The prescribed voltage should be reached after four minutes. In this manner, burning is avoided and a constantly high area growth of about 0.5 to 1.0 dm²/min is ensured. Immersion time depends on part size, rectifier capacity and the number of contact points. The nickel plating time should be 5-12 minutes, depending on the size and complexity of the parts. Further metal deposits then may be applied as required. The advantages of the direct-plate process in comparison to conventional processes for plating on plastics quite clearly outweigh the disadvantages. The reduction of the number of processing steps and rinsing baths shortens the pretreatment time from 50 to 20 minutes.
Practical Results
Since 2001, name signs and emblems made of ABS/PC used in the automobile industry have been treated in a plant with a tank volume of about 600 liters. The plant operates one shift per day. The cycle time is 7.5 minutes and the electroplated plastic area per goods carrier is 1.2 m²
At the end of January 2002, the throughput was about 4,000 m². The metallization test of large area automobile interior articles made of ABS/PC T85 was also conducted without any problems.
Enthone Inc.
| Table 1: | ||
|
Colloidal
|
New Direct-plate Process
|
Economical and
Ecological Advantages and Disadvantages |
| Etch 12 minutes, 68°C 400 g/l H2SO4 400 g/l CrO3 without catalyst |
Etch 5 minutes, 63°C 400 g/l H2SO4 320 g/l CrO3 |
• Reduced treatment time • Less production of Cr3+ • Less Cr drag-out • Catalyst cost |
| 4 Rinses | 4 Rinses | • Reduced waste water costs due to lower drag-out |
| Reduction 2 minutes, 30-50°C |
None | • No cost, no space required |
| 3 Rinses | None | • No cost, no space required |
| Pre-Immersion Dip in HCl | None | • No cost of chemicals, no effluent costs |
| Pd – Activator 4-5 min, 30°C | Activator 4 minutes, 18-22°C | • Complexed metal, Ammonium • Palladium-free • Possible need for cooling in summer |
| 3 Rinses | Conditioner 0.5 minutes | • Less effluent |
| Accelerator 2-3 minutes, 50°C |
Conducting Solution 1 min, room temperature |
• Less energy and effluent costs • No complexing or reducing agents • Unlimited operational life |
| 3 Rinses | 3 Rinses | • No complex treatment of effluent |
| Electroless Nickel 10 minutes, 30°C |
None | • No HNO3, no tank stripping • Reduced energy costs • No complexing agents • No expensive reduction agents • No make-ups due to high salt concentrations |
| 3 Rinses | None | • No waste water • Less space |
| ∑ 24 positions | ∑ 11 positions | • Less space needed |
| ∑ 48 minutes | ∑ 18 minutes | • Improved efficiency |
| Electrolytic Nickel and Electrolytic metal build up |
Electrolytic Nickel and Electrolytic metal build up |
• Electroplate to specification, no other line |
Related Content
A Chromium Plating Overview
An overview of decorative and hard chromium electroplating processes.
Read MoreAdvantages to Pumped Eductor Agitation
Not all agitation methods are created equally. Pumped agitation with eductor nozzles can improve process tanks and quickly show a reduction in operating costs while keeping staff safe, following environmental legislation and preventing pollution.
Read MoreTroubleshooting Alkaline Zinc
One of the most common problems that can arise when plating with alkaline zinc is an imbalance of brightener in the solution. In this helpful Ask the Expert article, Chad Murphy of Columbia Chemical discusses how different zinc metal concentrations and brightener concentrations can impact efficiency.
Read MoreProducts Finishing Reveals 2024 Qualifying Top Shops
PF reveals the qualifying shops in its annual Top Shops Benchmarking Survey — a program designed to offer shops insights into their overall performance in the industry.
Read MoreRead Next
Education Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read More














.jpg;maxWidth=300;quality=90)








