Pioneer Metal Finishing (Green Bay, Wisconsin) has grown its operation over its 75 years of business by opening new facilities, achieving new certifications and adding new capabilities to its lineup of surface engineering solutions. The company’s Medical Center of Excellence (CoE) facility in South Bend, Indiana, has expanded its capacity to serve the critical medical device industry, which enables Pioneer to deliver new medical innovations across its range of facilities in the U.S. and Mexico. PF recently spoke with Steve Smith, Corey Strege and Earl Lilly, Pioneer’s chief executive officer, chief commercial officer and South Bend general manager, respectively, to learn more about the Medical CoE and how it has impacted the company’s medical initiatives.
Pioneer Metal Finishing’s main focus for its medical customers is providing a complete surface-focused solution throughout the lifecycle of a medical device. Given the unique performance characteristics of medical device surfaces, bone integration with orthopedic implants as an example, Pioneer says it is critical to collaborate with medical device customers early in the product development process in order to optimize the targeted clinical solution. Supporting these same customers during product launch, manufacturing scale up and through the lifetime of the product is an essential component of Pioneer’s Medical CoE.
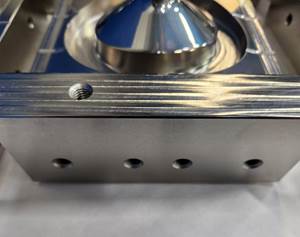
Pioneer’s primary product offerings today focus on sterilization resilient aluminum anodizing solutions and high-performance wear and lubricity surfaces. The targeted medical devices – many of which are aluminum-based – can be found in sterilization cases and trays, patient handling beds, jigs and fixtures in medical tools, external fixators for broken limbs, as well as components for robotics and minimally invasive surgeries. Pioneer also works on components found in critical imaging systems, cameras and MRI and X-ray machines. Strege adds, “We have specialized coatings for functional performance, like tribological management or wear and lubricity enhancement, and optical characteristics mainly in management of light and heat.”
The capabilities of Pioneer’s Medical CoE include fully automated type II and III anodizing lines, electroless nickel (EN) lines, conversion coatings, mechanical finishing and more.
Photo Credit: Pioneer Metal Finishing
Pioneer’s Medical CoE features fully automated type II and III anodizing lines, electroless nickel (EN) lines, conversion coatings, mechanical finishing, part marking and graphics, passivation stainless steel treatments, a number of proprietary engineered finishes and a fully established testing lab to verify product quality. Strege notes one of Pioneer’s key capabilities is developing and matching anodizing colors, which are used to finish sterilization trays and cases. Through manufacturing process validation, the company can match colors and produce them consistently through the life of the product. With medical customers requiring high tolerance consistency of anodized products in every color of the rainbow, Strege says, “This is another unmet need in the medical device space.”
ISO Certification and Collaboration Drives Innovation
Pioneer’s targeted medical devices can be found in sterilization cases and trays, patient handling beds, jigs and fixtures in medical tools, external fixators for broken limbs, as well as components for robotics and minimally invasive surgeries. Photo Credit: Pioneer Metal Finishing
Pioneer’s South Bend facility recently achieved ISO 13485 certification, a rigorous standard for medical device manufacturers that requires them to demonstrate the effectiveness of their quality management systems, which is especially important for anything in or around the operating room. The certification was earned after about two years of work spent honing its processes to pass a meticulous audit of its facility, which Lilly comments is “a completely different level of scrutiny.” He adds, “13485 gets us a seat at the table with medical device customers by establishing a similar level of operational rigor they are held to by the FDA. It is key in helping these customers effectively manage the risks associated with medical device manufacturing.”
ISO certification signifies growth for its South Bend facility, but Smith is clear that it is also part of a wider effort in Pioneer’s journey of continuous improvement across all its facilities. Smith explains, “When we take an initiative as an organization, it is never about an individual location. It is about our ability to combine an enterprise of solutions.”
Pioneer finishes critical devices for a range of medical applications. Photo Credit: Pioneer Metal Finishing
Achieving the ISO 13485 certification was phase one for Pioneer’s Medical CoE. Phase two is to launch new products and collaborate with industry peers and customers to drive future innovations for the medical device industry. Pioneer has its eyes on more collaboration moving forward, and Strege adds that over the last six months, he has noticed “a significant escalation of requests for collaboration on new product development. People are bringing their problems to us more frequently.”
With ISO 13485 certification in hand, Pioneer is currently working on a product that Strege says is the “culmination of two-and-a-half years of work to identify and improve upon key unmet needs in the med device space as it relates to surface engineering.” According to Strege, one of these needs is providing an aluminum anodizing product that can withstand or be resilient to two types of sterilization: steam autoclave and vaporized hydrogen peroxide (VHP). Currently, there is no product on the market that satisfies both types of sterilization over repeated use at high volumes. More will be revealed in Pioneer’s booth at the OMTEC 2022 orthopedic manufacturing expo in Rosemont, Illinois, but Strege discloses, “Our goal is getting towards a product that is sterilization resilient up to 300 autoclave or up to 300 VHP cycles.”
Related Content
Top Shop Builds Original Systems for Coating Medical Devices
Engineers at Surgical Coatings in Colorado have been ingeniously developing their own equipment, automation, processes and software since this powder coater’s inception in 1995.
Read MorePrecision Coating Receives ISO 14001 Certification
The company says its commitment to the environment and its health led it to seek this certification.
Read MoreEngineered Coatings Offer Improvement for Medical Device Manufacturers
Diamond electroless nickel coating provides better lubricity and improved wear resistance for molds, resulting in better quality and productivity.
Read MoreMasking Solutions for Medical Applications
According to Custom Fabricating and Supplies, a cleanroom is ideal for converting, die cutting, laminating, slitting, packaging and assembly of medical-grade products.
Read MoreRead Next
Pioneer Achieves Medical Device Certification
Pioneer Metal Finishing’s South Bend, Indiana based Medical Center of Excellence facility has achieved ISO 13485 medical device certification.
Read MorePioneer Metal Finishing Wins Efficiency Award
Pioneer Metal Finishing in Warren, Michigan, won the Macomb County Business Awards' Efficiency Expert award for reducing its environmental footprint and utility usage.
Read MorePioneer Metal Finishing Employees Give Back to Communities
Pioneer Metal Finishing employees donated more $12,000 to local charities and more than 7,200 pounds of food that were distributed to food pantries in their communities.
Read More


















.jpg;maxWidth=300;quality=90)